Category: Dystonia: Pathophysiology, Imaging
Objective: Whole-brain metabolic network analysis was performed to 1) identify the symptomatic network of faciobrachial dystonic seizures (FBDS); 2) to explore independent neuroimaging predictors; and 3) elucidate the functional coupling of the neocortex-subcortex-cerebellum circuit in FBDS.
Background: Faciobrachial dystonic seizures (FBDS) is the distinct clinical features of autoimmune encephalitis (AE) caused by antibodies against leucine-rich glioma-inactivated 1 (LGI1). Typical FBDS are described as sudden, brief, lateralized tonic contractions (or “spasms”) involving the upper limb, the ipsilateral face, and less frequently the leg, and are often associated with hand dystonia. They can be unilateral, bilateral asynchronous, or alternating with short delays and tend to recur several times per day. The distinct characteristics of FBDS are easily recognizable, but its nature (epileptic seizure vs. paroxysmal movement disorder) and origin (cortical vs. subcortical) remain debated. The pathophysiological pattern and neural mechanisms underlying the symptoms remain largely unexplored.
Method: We included 30 patients with anti-LGI1 AE and 30 controls from a retrospective observational cohort (Table 1). We performed whole-brain metabolic pattern analysis to assess the symptomatic networks in FBDS in patients with anti-LGI1 AE. Logistic regression was applied to explore independent predictors of FBDS and Pearson correlation was used to evaluate the functional coupling within brain regions.
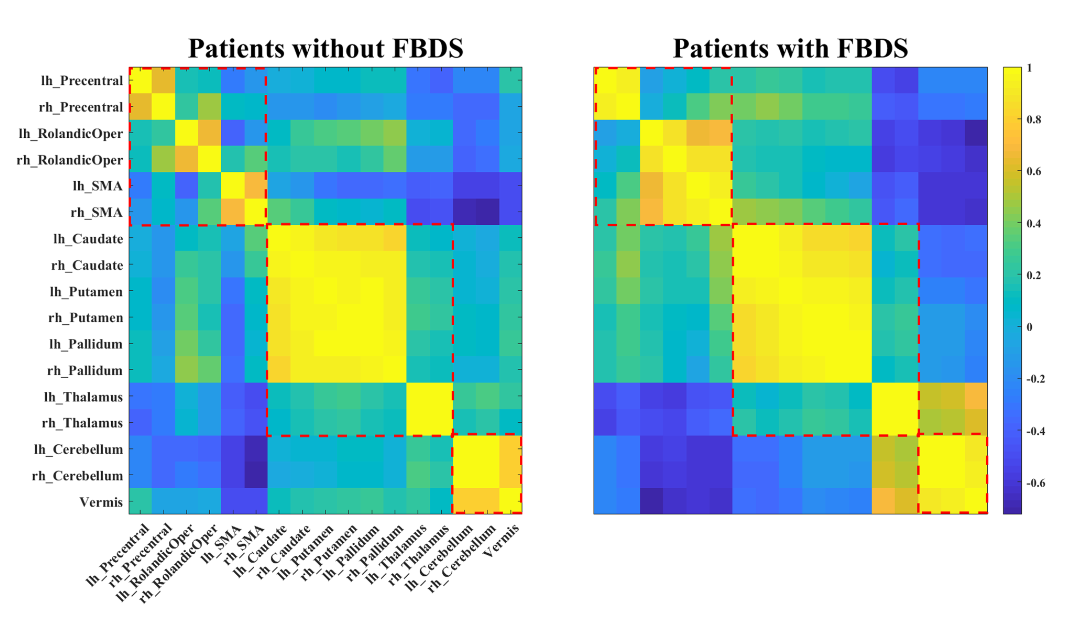
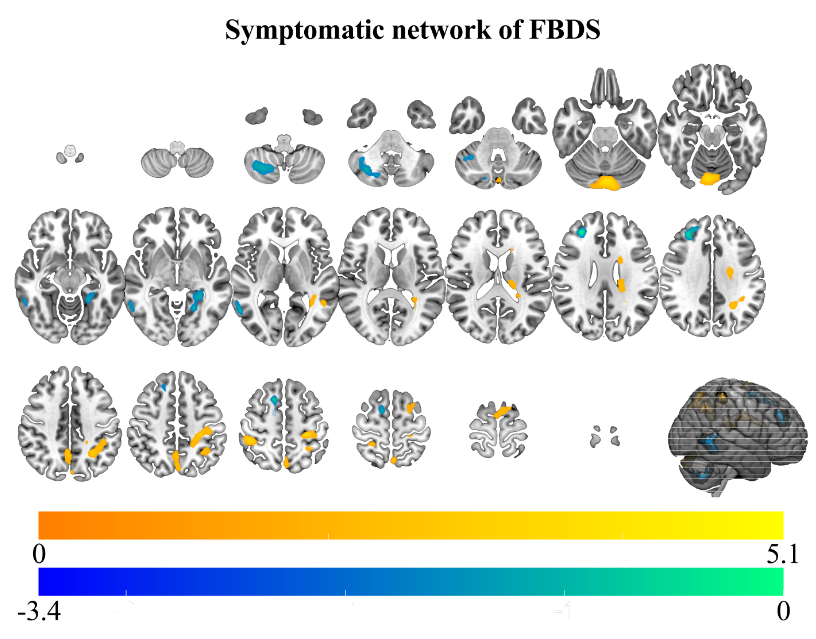
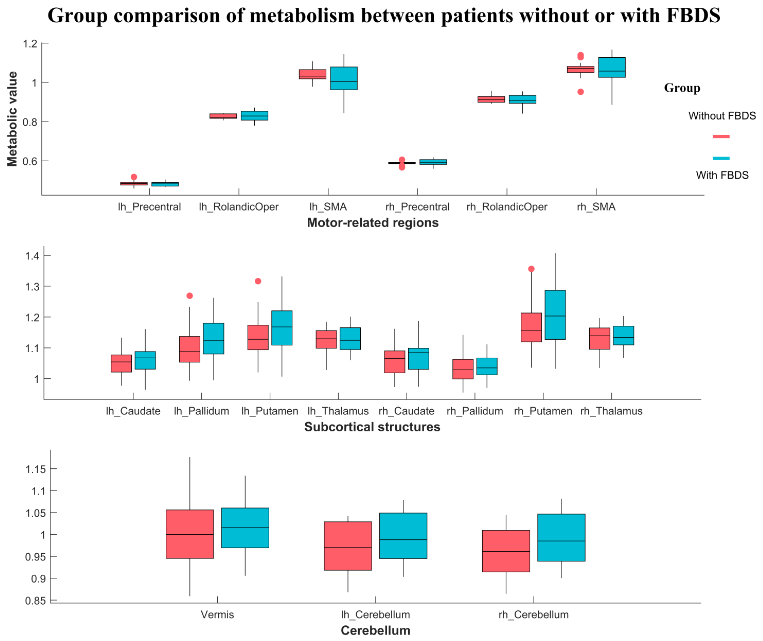
Results: The symptomatic network of FBDS mainly involved the Rolandic area, subcortical structures (caudate nucleus and thalamus), and the cerebellum (Figure 1). Hypermetabolism in the cerebellum was an independent predictor of FBDS (P < 0.01) (Table 2). Moreover, stronger functional coupling within motor-related brain regions, intra-subcortical structures, and the cerebellum was observed in patients with FBDS (Figure 2&3).
Conclusion: Our study identified the symptomatic network of FBDS in patients with anti-LGI1 AE, which may contribute to our understanding of the neural mechanisms underlying FBDS and improve future diagnosis and treatment.
References: [1] Dalmau J, Graus F. Antibody-Mediated Encephalitis[J]. The New England journal of medicine, 2018, 378(9): 840-851.
[2] van Sonderen A, Petit-Pedrol M, Dalmau J, Titulaer MJ. The value of LGI1, Caspr2 and voltage-gated potassium channel antibodies in encephalitis[J]. Nature reviews Neurology, 2017, 13(5): 290-301.
[3] Morano A, Fanella M, Giallonardo AT, Di Bonaventura C. Faciobrachial Dystonic Seizures: The Borderland Between Epilepsy and Movement Disorders[J]. Movement disorders clinical practice, 2020, 7(2): 228-229.
[4] Chen C, Wang X, Zhang C, Cui T, Shi WX, Guan HZ, Ren HT, Shao XQ. Seizure semiology in leucine-rich glioma-inactivated protein 1 antibody-associated limbic encephalitis[J]. Epilepsy & behavior : E&B, 2017, 77: 90-95.
To cite this abstract in AMA style:
J. Mo, K. Zhang, X. Shao. Whole-brain metabolic pattern analysis of faciobrachial dystonic seizures (FBDS) [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/whole-brain-metabolic-pattern-analysis-of-faciobrachial-dystonic-seizures-fbds/. Accessed October 17, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/whole-brain-metabolic-pattern-analysis-of-faciobrachial-dystonic-seizures-fbds/