Category: Parkinson's Disease: Neuroimaging
Objective: This study aims to explore the white matter structural network associated with motor reserve (i.e., individual capacity to cope with nigrostriatal dopamine depletion) in patients with newly diagnosed Parkinson’s disease (PD).
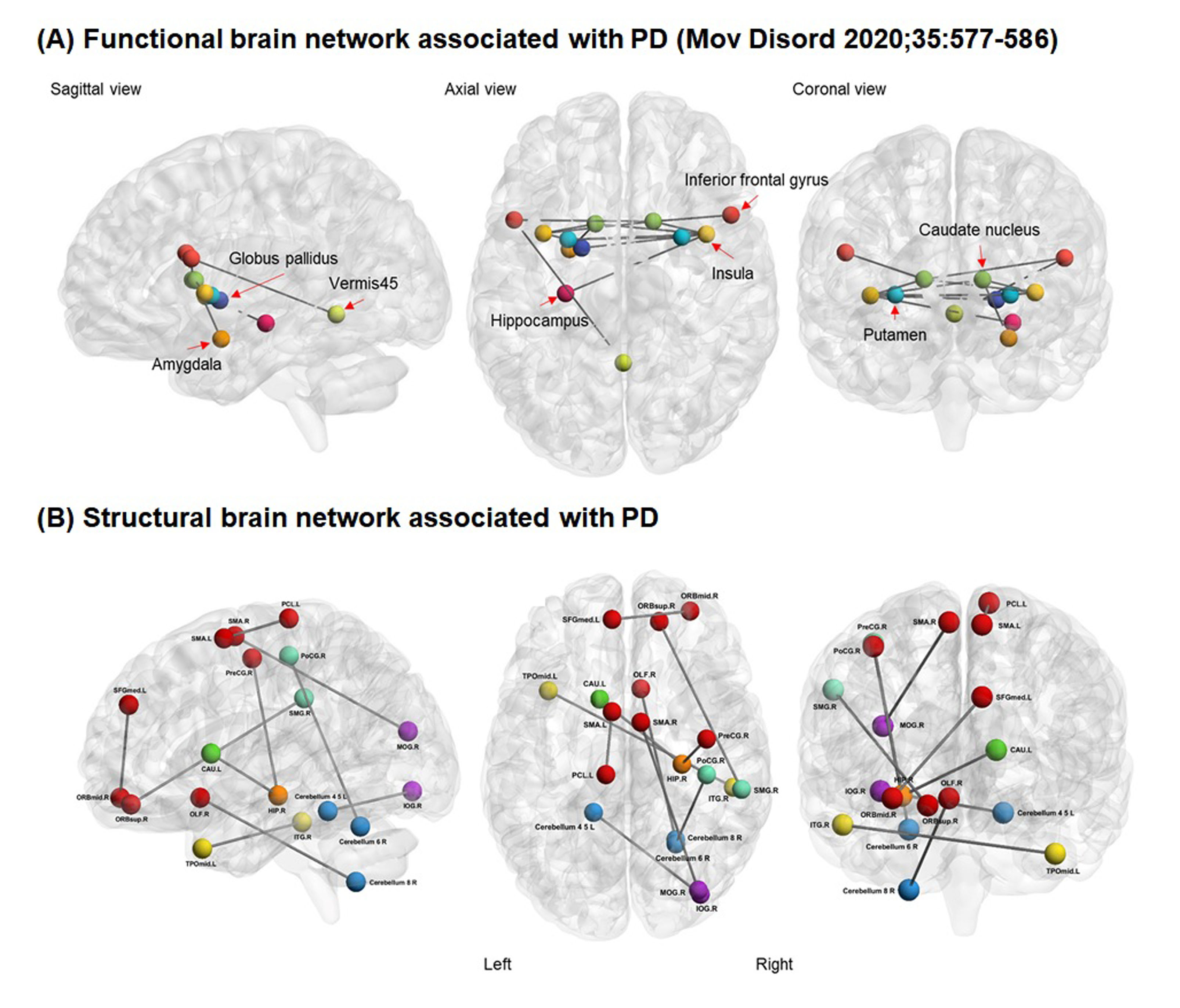
Background: The concept of motor reserve was recently introduced to explain the individual variability in parkinsonian motor deficits despite similar degrees of striatal dopamine deficits in patients with PD [1-4]. Some evidence suggests that initial motor reserve can be maintained with disease progression and affect the long-term motor prognosis [2, 3, 5], and enhancing motor reserve may be a reasonable disease-modifying therapeutic strategy in PD [1]. Previously, we identified the functional brain network associated with motor reserve [2], which comprised the basal ganglia, inferior frontal cortex, insula, and cerebellar vermis. In this study, we hypothesized that structural brain network associated with motor reserve would also exist in PD.
Method: We enrolled 238 patients with newly diagnosed PD who underwent 18F-FP-CIT PET and brain MRI including diffusion tensor imaging at initial assessment. We estimated individual motor reserve based on the Unified Parkinson’s Disease Rating Scale Part III (UPDRS-III) scores and dopamine transporter availability in the posterior putamen using a residual model as described previously [2]. We then performed threshold-free network-based statistics (TFNBS) analysis to identify the structural brain network associated with the motor reserve estimates.
Results: The mean age at PD symptom onset was 69.1 years and the mean UPDRS-III score at initial assessment (i.e., drug-naive status) was 22.4. TFNBS analysis identified the structural network in which structural connectivity positively correlated with the motor reserve estimates, including 19 nodes mainly in the frontal region and cerebellum. Given that the frontal area and cerebellum are associated with motor deficits in PD, this neuroanatomical relevance reinforced the robustness of our findings on the structural correlates of motor reserve. Meanwhile, no significant networks were detected in which structural connectivity negatively correlated with the motor reserve estimates.
Conclusion: We identified the structural brain network associated with motor reserve in patients with PD. Connectivity strength within the identified network would indicate an individual’s capacity to tolerate PD-related pathologies.
References: 1. Chung SJ, Lee JJ, Lee PH, Sohn YH. Emerging Concepts of Motor Reserve in Parkinson’s Disease. J Mov Disord 2020; 13: 171-184.
2. Chung SJ, Kim HR, Jung JH, Lee PH, Jeong Y, Sohn YH. Identifying the Functional Brain Network of Motor Reserve in Early Parkinson’s Disease. Mov Disord 2020; 35: 577-586.
3. Chung SJ, Yoo HS, Lee YH, Lee HS, Lee PH, Sohn YH. Initial motor reserve and long-term prognosis in Parkinson’s disease. Neurobiol Aging 2020; 92: 1-6.
4. Chung SJ, Lee PH, Sohn YH, Kim YJ. Glucocerebrosidase Mutations and Motor Reserve in Parkinson’s Disease. J Parkinsons Dis 2021; 11: 1715-1724.
5. Lee PC, Artaud F, Cormier-Dequaire F, et al. Examining the Reserve Hypothesis in Parkinson’s Disease: A Longitudinal Study. Mov Disord 2019.
To cite this abstract in AMA style:
S. Chung, Y. Kim, C. Park, H. Shin, H. Lee, Y. Kim, M. Yun, P. Lee, Y. Sohn, Y. Jeong. White matter structural network of motor reserve in early Parkinson’s disease [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/white-matter-structural-network-of-motor-reserve-in-early-parkinsons-disease/. Accessed April 26, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/white-matter-structural-network-of-motor-reserve-in-early-parkinsons-disease/