Category: Other
Objective: To investigate the frequency of genetic variants in dystonia (DYT) genes in patients with PD and vice versa.
Background: The impact of genetic variants on phenotypic expression is difficult to predict and ranges from high disease-related effects for pathogenic variants via low effects of risk variants to no (yet known) effect for benign variants. Comparative studies of variant frequencies between affected and unaffected carriers usually help in variant interpretation. However, the situation is further complicated by the phenomena of reduced penetrance (being healthy despite carrying a pathogenic variant) and variable expressivity (e.g., dystonia-causing variants being present in patients with Parkinson´s disease (PD)).
Method: The ProMoveGene sample, including individuals with PD, DYT, and controls, was genotyped using the Global Screening Array (GSA, Illumina) containing a custom content covering ~1000 rare variants in PD- or DYT-related genes. Another ~1,000 individuals with early-onset or familial PD were analyzed using short-read genome sequencing as part of the Global Parkinson’s Genetics Program (GP2) [1] and screened for rare variants in known DYT (TOR1A, THAP1, GNAL, ANO3, KMT2B, PRKRA, GCH1, and SGCE) or PD (GBA1, LRRK2, SNCA, VPS35, PINK1, PRKN, and PARK7) genes. In contrast to the GSA hot spot screening, all genetic variants in these genes were targeted with genome sequencing. Only (likely) pathogenic variants and variants of uncertain significance (VUS) were considered.
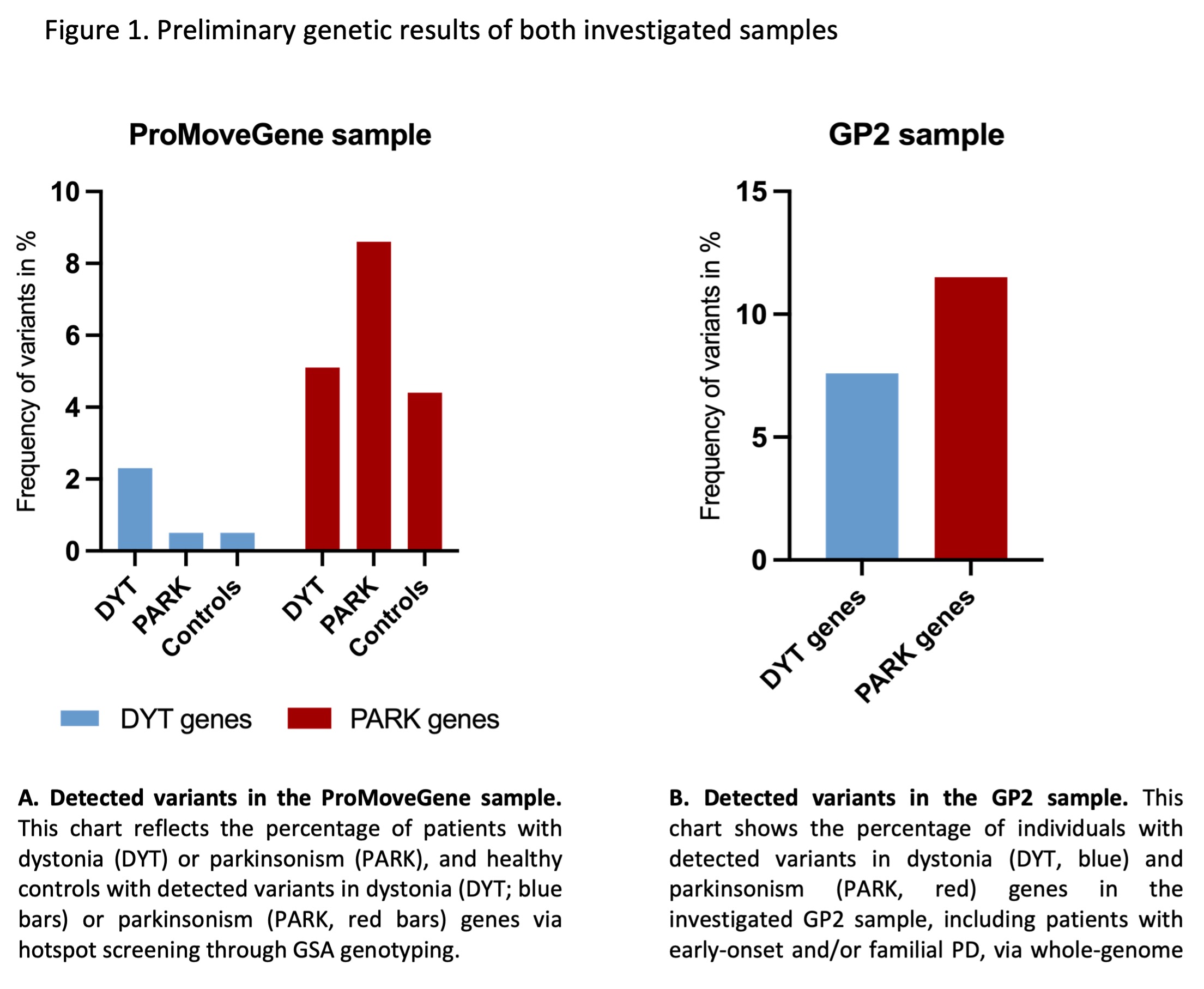
Results: The ProMoveGene sample includes ~2,600 individuals with PD, ~4,400 with DYT, and ~5,800 controls. Variants in DYT genes were most frequent in individuals with DYT (2.3%), but also a small percentage of PD patients and controls carried variants in DYT genes (0.5% each). While variants in PD genes were most frequent in PD patients (8.6%), a considerable number was also detected in DYT patients (5.1%), and controls (4.4%) (Figure 1A).
Of the ~1,000 patients with early-onset/familial PD from GP2, 7.6% carried (likely) pathogenic variants or VUS in DYT and 11.5% in PD genes (Figure 1B); controls were not yet analyzed.
Conclusion: Variants in DYT genes can be found in patients with DYT but also in patients with PD and vice versa, and in controls, the latter may correspond to reduced penetrance or in case of VUS, are not disease-causing. The impact of these variants needs to be further investigated in even larger sample sizes.
References: 1. Global Parkinson’s Genetics, P., GP2: The Global Parkinson’s Genetics Program. Mov Disord, 2021. 36(4): p. 842-851.
To cite this abstract in AMA style:
L. Lange, A. Illarionova, K. Grütz, EJ. Vollstedt, B-H. Laabs, S. Löns, G. Kilic-Berkmen, F. Hinrichs, H. Padlock, L. Screven, T. Bäumer, H. Jinnah, N. Brüggemann, Z-H. Fang, K. Lohmann, C. Klein. Variant frequencies in dystonia and Parkinson’s disease genes cross phenotypic boundaries [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/variant-frequencies-in-dystonia-and-parkinsons-disease-genes-cross-phenotypic-boundaries/. Accessed January 7, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/variant-frequencies-in-dystonia-and-parkinsons-disease-genes-cross-phenotypic-boundaries/