Category: Parkinson’s Disease: Clinical Trials
Objective: To assess long-term treatment switch patterns and discontinuation rates in patients (pts) with Parkinson’s disease (PD) treated with foslevodopa/foscarbidopa (LDp/CDp) mono- or combination therapy.
Background: PD progression can increase treatment complexity, leading to polypharmacy and higher burden of care. LDp/CDp, a formulation of levodopa/carbidopa (LD/CD) prodrugs delivered as a 24-hour/day continuous subcutaneous infusion, led to improved motor symptoms [1,2]. The long-term sustainability of LDp/CDp as monotherapy warrants further study.
Method: This post hoc analysis assessed pts with PD who had ≥2.5 “Off” hours/day and received LDp/CDp as mono- and/or combination therapy in 2 trials: a phase 3, single-arm, 52‑week, open‑label trial (NCT03781167; 4-week optimization period in which baseline [BL] concomitant PD medication could be adjusted to achieve optimal clinical response, followed by a 48-week maintenance period) and the 96-week open-label extension (OLE; NCT04379050) of the parent trial. Monotherapy was defined as LDp/CDp use without any concomitant PD medications. Combination therapy allowed concomitant PD medication use (not LD/CD or COMT inhibitors). The LDp/CDp regimen used in the parent trial could be sustained (as mono- or combination therapy) or modified (mono- to combination therapy or vice versa) at the investigator’s discretion at OLE BL.
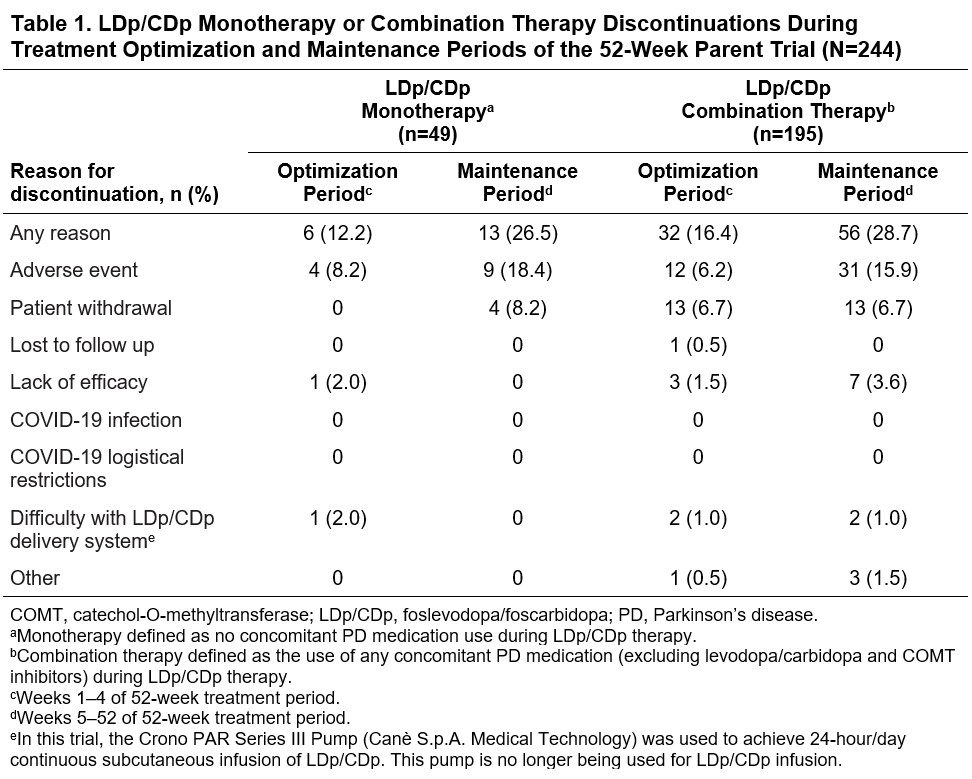
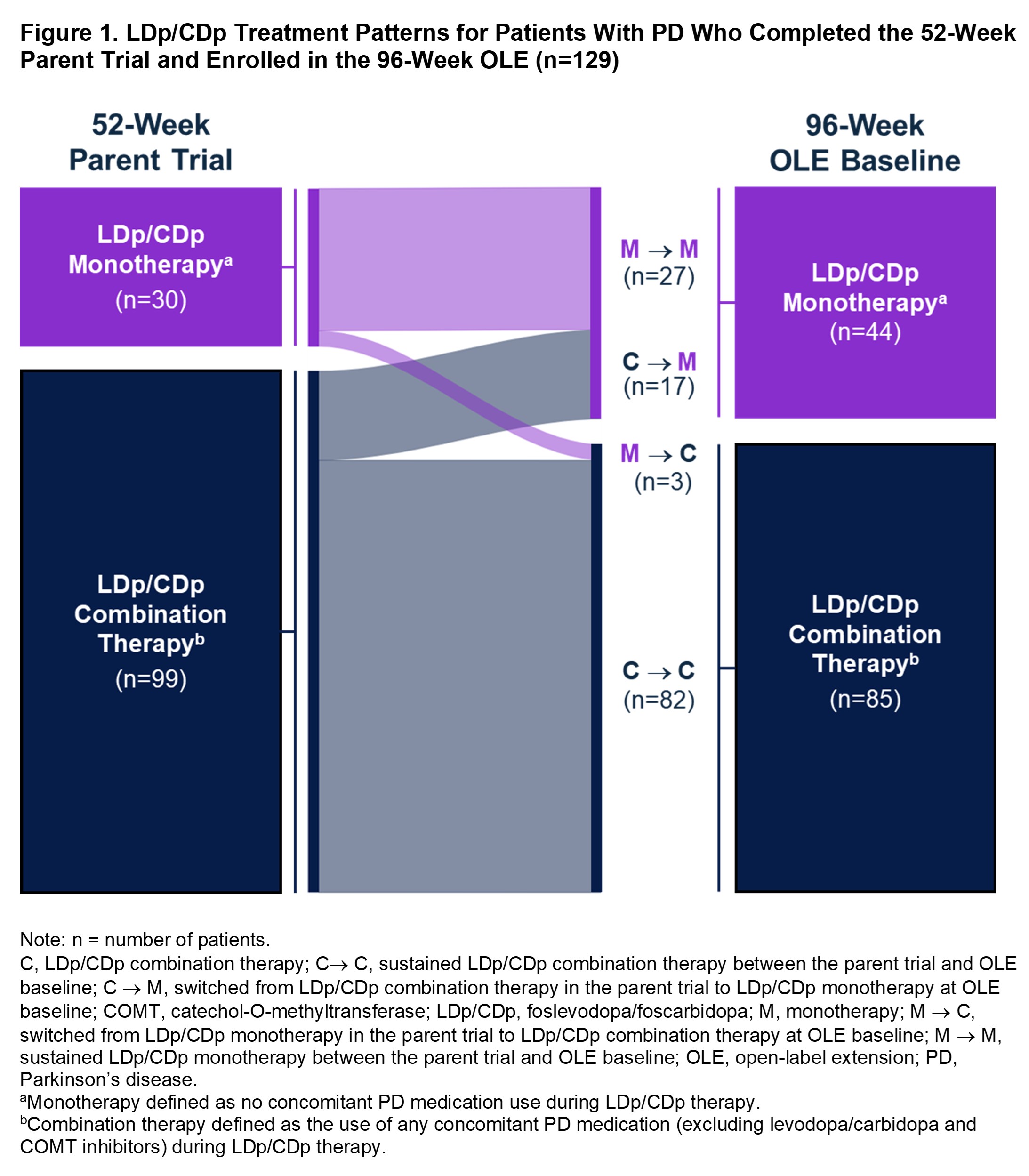
Results: A total of 244 pts enrolled in the parent trial (LDp/CDp monotherapy, n=49; combination therapy, n=195). The discontinuation rate was numerically lower with monotherapy (38.8%; n=19) vs combination therapy (45.1%; n=88) [table1]. Of the 137 pts who completed the parent trial, 129 enrolled in the OLE (n=30 and n=99 assigned to LDp/CDp mono- and combination therapy during the parent trial, respectively, vs n=44 and n=85 at OLE BL; [figure1]). Most pts (90.0%; n=27/30) who initiated LDp/CDp as monotherapy continued with monotherapy into the OLE [figure1]. Almost one-fifth (17.2%; n=17/99) of pts who initiated LDp/CDp as combination therapy discontinued all concomitant PD medications (i.e. switch to LDp/CDp monotherapy) by OLE BL; the remaining 82.8% (n=82/99) maintained combination therapy between parent trial and OLE BL [figure1].
Conclusion: Most pts with PD treated with LDp/CDp as monotherapy can continue without adjunctive PD medications for up to 48 weeks, and patients on LDp/CDp combination therapy may be switched to LDp/CDp monotherapy over time.
Table 1
Figure 1
References: 1. Soileau MJ, et al. Lancet Neurol. 2022;21:1099–1109.
2. Aldred J, et al. Neurol Ther. 2023;12:1937–1958.
To cite this abstract in AMA style:
J. Aldred, T. Henriksen, M. Bouchard, J. Martínez-Castrillo, M. Soileau, D. Standaert, L. Bergmann, R. Gupta, P. Kukreja, M. Shah, S. Isaacson. Treatment Patterns With Long-Term Foslevodopa/Foscarbidopa Use in Parkinson’s Disease [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/treatment-patterns-with-long-term-foslevodopa-foscarbidopa-use-in-parkinsons-disease/. Accessed January 6, 2026.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/treatment-patterns-with-long-term-foslevodopa-foscarbidopa-use-in-parkinsons-disease/