Category: Parkinson’s Disease: Clinical Trials
Objective: To assess whether a time-to-event (TTE) approach may mitigate the masking effect of changing symptomatic therapy on Movement Disorders Society–Unified Parkinson’s Disease (PD) Rating Scale (MDS-UPDRS) Part III.
Background: In clinical trials of potential disease-modifying treatments, improvement due to change in symptomatic therapies may mask disease progression as measured by MDS-UPDRS Part III and thus interfere with the ability to detect treatment effects. A change in therapy that is disproportionately higher in the placebo arm may lead to underestimation of the treatment effect if measured as continuous change from baseline (CFB). A TTE approach might mitigate such masking effects as events may be reached prior to change in medication.
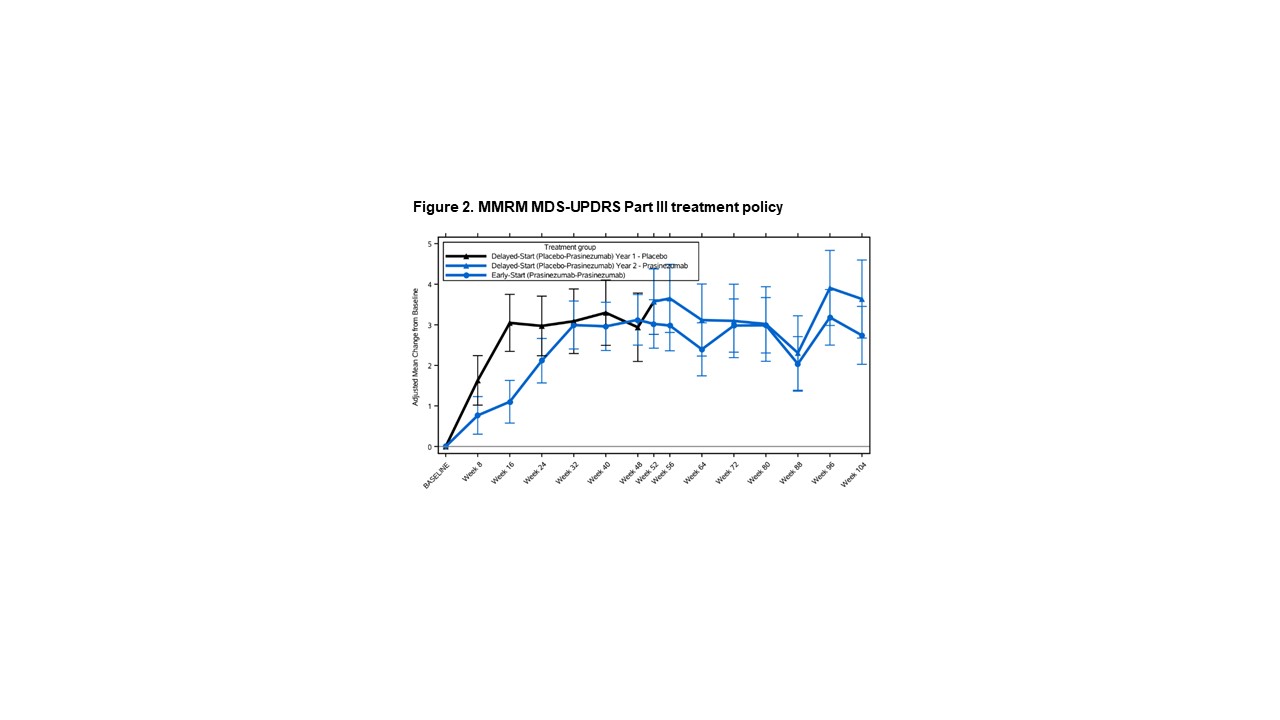
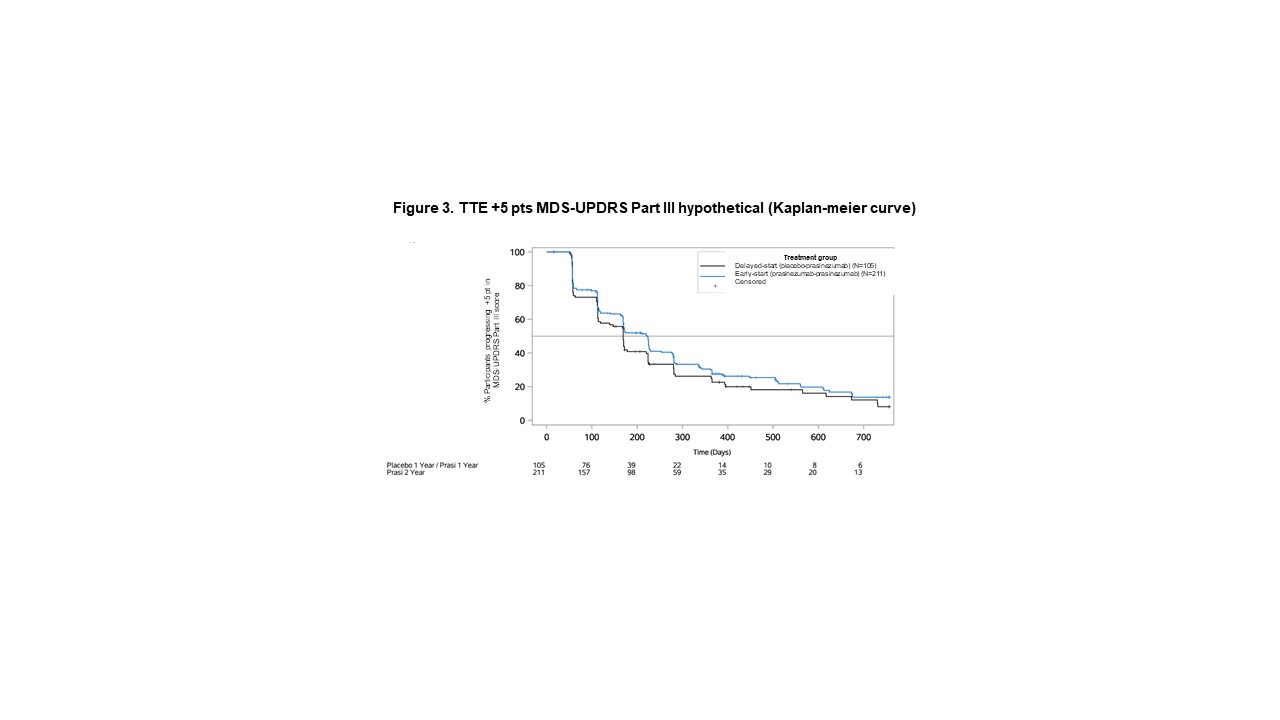
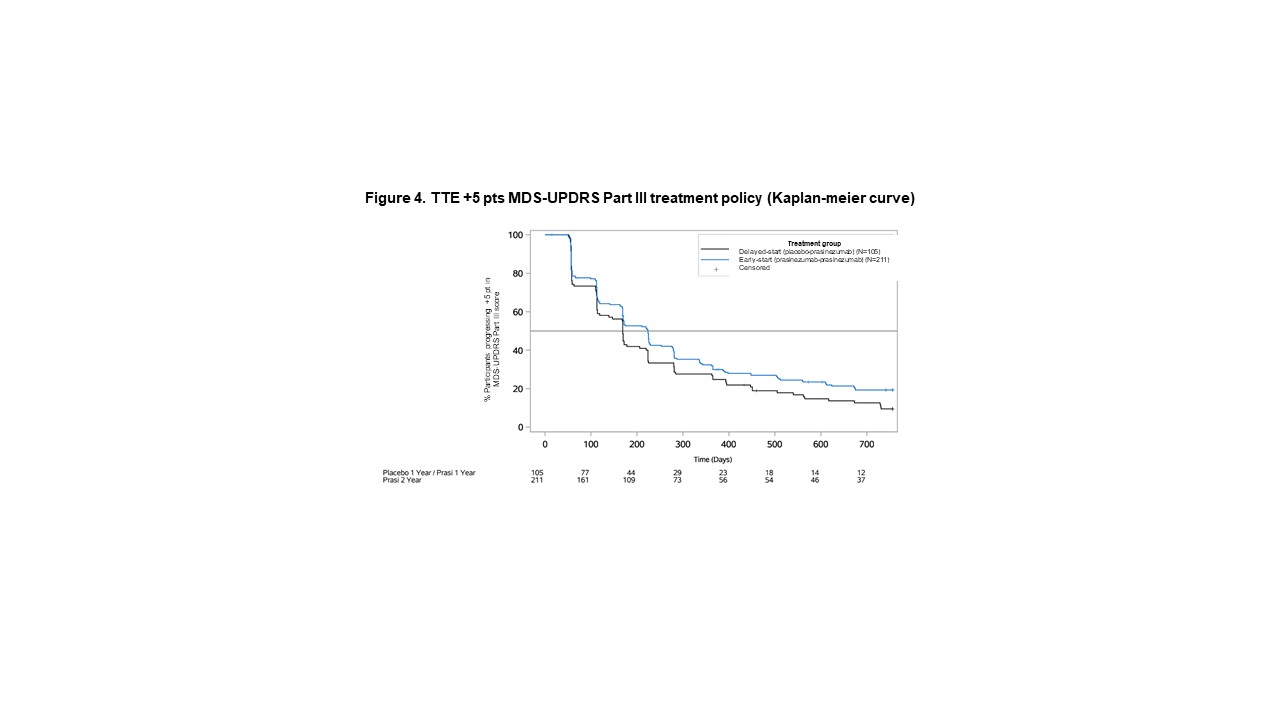
Method: In this exploratory post-hoc analysis of the PASADENA Phase II trial, differences between early-start (receiving prasinezumab for 104 weeks) and delayed-start (receiving placebo for 52 weeks followed by prasinezumab for 52 weeks) groups were evaluated for the continuous MDS-UPDRS Part III CFB at Week 104 using a Mixed Models for Repeated Measures, and for the TTE to +5 points in MDS-UPDRS Part III [1] using Cox proportional hazards model. Continuous and TTE endpoints were analysed conventionally, including all data points regardless of change in symptomatic therapy (treatment policy strategy), and by extrapolation or censoring using all data points prior to change in symptomatic therapy (hypothetical estimand strategy).
Results: Data from 309 participants were analysed. For the continuous analysis, the mean±standard error CFB at Week 104 was 10.59±1.41 points for delayed-start, and 8.42±1.00 for early start group using hypothetical strategy (difference [80% CI] -2.17 [-4.37, 0.03]), and 3.55±0.96 for delayed-start, and 2.72±0.71 for early-start group using treatment policy (difference -0.83 [-2.33, 0.67]). For the TTE analysis, the hazard ratio [80%CI] between delayed and early start groups was 0.82 [0.69, 0.98] using hypothetical and 0.77 [0.65, 0.91] using treatment policy strategy.
Conclusion: Progression in motor signs was larger under hypothetical compared with treatment policy strategy, suggesting a masking effect of symptomatic therapy. The consistent results under either estimand strategy suggest that a TTE analysis may mitigate the potential masking effect of symptomatic therapy on MDS-UPDRS Part III in PD.
References: 1. Horváth K, Aschermann Z, Ács P, Deli G, Janszky J, Komoly S, Balázs É, Takács K, Karádi K, Kovács N. Minimal clinically important difference on the Motor Examination part of MDS-UPDRS. Parkinsonism & related disorders. 2015 ;21(12):1421-6.
To cite this abstract in AMA style:
S. Zanigni, N. Shariati, T. Simuni, N. Pavese, K. Marek, R. Postuma, A. Monnet, E. Moore, A. Hahn, E. Davies, N. Pross, D. Trundell, T. Nikolcheva, G. Pagano. Time-to-event approach mitigates the treatment masking effect of symptomatics on MDS-UPDRS Part III [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/time-to-event-approach-mitigates-the-treatment-masking-effect-of-symptomatics-on-mds-updrs-part-iii/. Accessed April 15, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/time-to-event-approach-mitigates-the-treatment-masking-effect-of-symptomatics-on-mds-updrs-part-iii/