Session Information
Date: Tuesday, September 24, 2019
Session Title: Parkinsonisms and Parkinson-Plus
Session Time: 1:45pm-3:15pm
Location: Agora 3 West, Level 3
Objective: Impel NeuroPharma has developed the patient-friendly, self or caregiver actuatedPrecision Olfactory Delivery (IMPEL PODTM) device to achieve consistent upper nasal cavity drug delivery, rapid systemic uptake and higher bioavailability relative to standard nasal sprays. This study with L-dopa for treatment of OFF episodes of Parkinson’s Disease (PD) assessed safety, tolerability, pharmacokinetics (PK) and pharmacodynamics of L-dopa administered with a dopa decarboxylase inhibitor (DCI).
Background: As PD progresses, the brain requires more frequent and higher doses of L-dopa, yet still patients suffer disabling OFF episodes due to L-dopa (the “platinum” standard treatment for PD1) plasma fluctuations. The IP is a drug-device combination product of novel formulations of L-dopa delivered to the upper nasal cavity by the POD device.
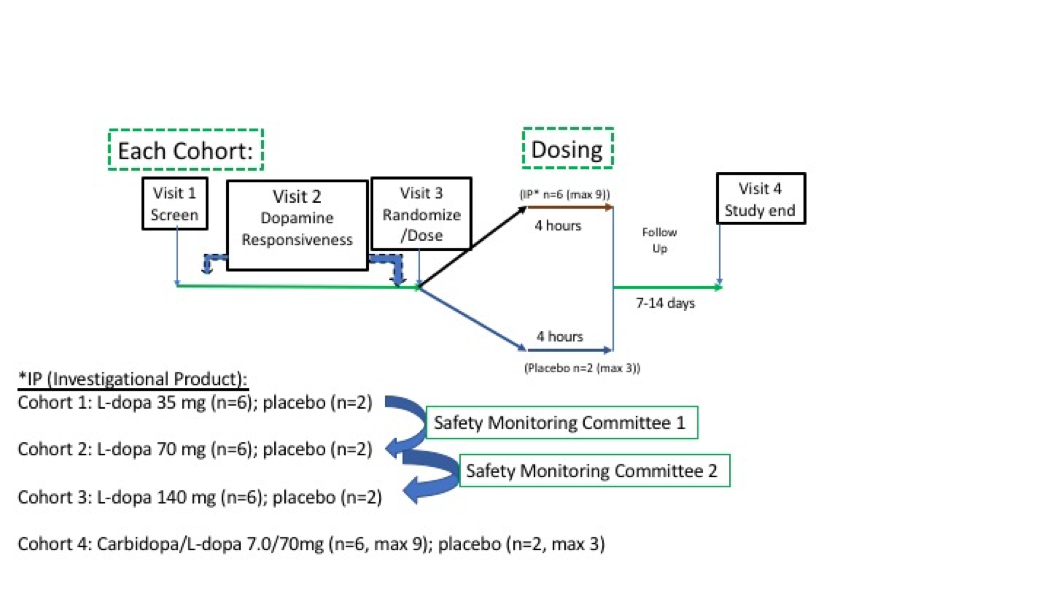
Method: A randomized, placebo controlled, single dose study was conducted in L-dopa-responsive PD patients in an OFF episode at 5 Movement Disorder Clinics with safety, tolerability and PK blood collections for 2 hours post dosing and repeated MDS-UPDRS assessments. Doses of 35 mg, 70 mg and 140 mg per dose were assessed, pre-dosed with oral benserazide. In a final additional cohort, a novel formulation of carbidopa/L-dopa 7/70mg (without oral DCI) was tested [Figure 1].
Results: The first 3 cohorts have completed dosing. Blinded, drug related TEAE data from the first 2 cohorts of 12 active and 4 placebo dosed subjects revealed single episodes of: hypertension, sinus dryness, foggy head, mucus back of nose/throat, sneezing + coughing, drowsiness and nasal irritation and 2 episodes of headache. All AEs were mild, self-limiting and most lasted less than 1 hour. There were 3 (blinded) reports of slight post dosing dyskinesia in cohort 1 (at 120 minutes or later), but no reports in cohort 2.
Conclusion: Satisfactory safety and tolerability results have allowed for 3 escalating doses of IP to be administered to PD patients in morning OFF episodes, and a further cohort planned with combined carbidopa/L-dopa, with detailed collection and analysis of PK and pharmacodynamic data ongoing.
References: 1LeWitt PA. Levodopa therapy for Parkinson’s Disease: Pharmacokinetics and Pharmacodynamics. Movement Disorders 2015; 30: 64-72.
To cite this abstract in AMA style:
S. Shrewsbury, J. Campbell, M. Swardstrom, A. Lehn, K. Satterly, J. Hoekman. THOR 201: A Proof-of-Concept Study Assessing Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of L-dopa delivered by Impel’s Precision Olfactory Delivery (PODTM) to Parkinson’s Disease Patients in an OFF Episode (in the presence of Dopa Decarboxylase Inhibitor) [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/thor-201-a-proof-of-concept-study-assessing-safety-tolerability-pharmacokinetics-and-pharmacodynamics-of-l-dopa-delivered-by-impels-precision-olfactory-delivery-podtm-to-parkinson/. Accessed December 12, 2025.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/thor-201-a-proof-of-concept-study-assessing-safety-tolerability-pharmacokinetics-and-pharmacodynamics-of-l-dopa-delivered-by-impels-precision-olfactory-delivery-podtm-to-parkinson/