Category: Parkinson's Disease: Cognitive functions
Objective: To test the hypothesis that antidromic activation of the hyperdirect pathway might mediate the effects of STN-DBS on movement initiation control.
Background: In PD, STN-DBS improves movement initiation but also worsens executive functions and favors impulsive behavior. The mechanisms of action of STN-DBS accounting for these effects are still unclear. Beyond orthodromic activation, antidromic activation of the hyperdirect pathway is also likely. The induced modulation of SMA activity, which is a central node of the inhibitory control network, could account for changes in the ability to initiate movements. This hypothesis assumes that the behavioral effect of DBS is a function of the strength of structural connectivity between the stimulated area and the SMA.
Method: 19 patients with idiopathic PD implanted with bilateral STN-DBS were evaluated ON and OFF-DBS using a simple Go-NoGo task. Using the LeadDBS toolbox [1], we localized the active contacts and modeled the Volumes of Activated Tissue (VAT; N=15 patients). Then, the strength of the structural connectivity between the VAT and the SMA was estimated based on a normative structural connectome [2], and correlated to the behavioral variables.
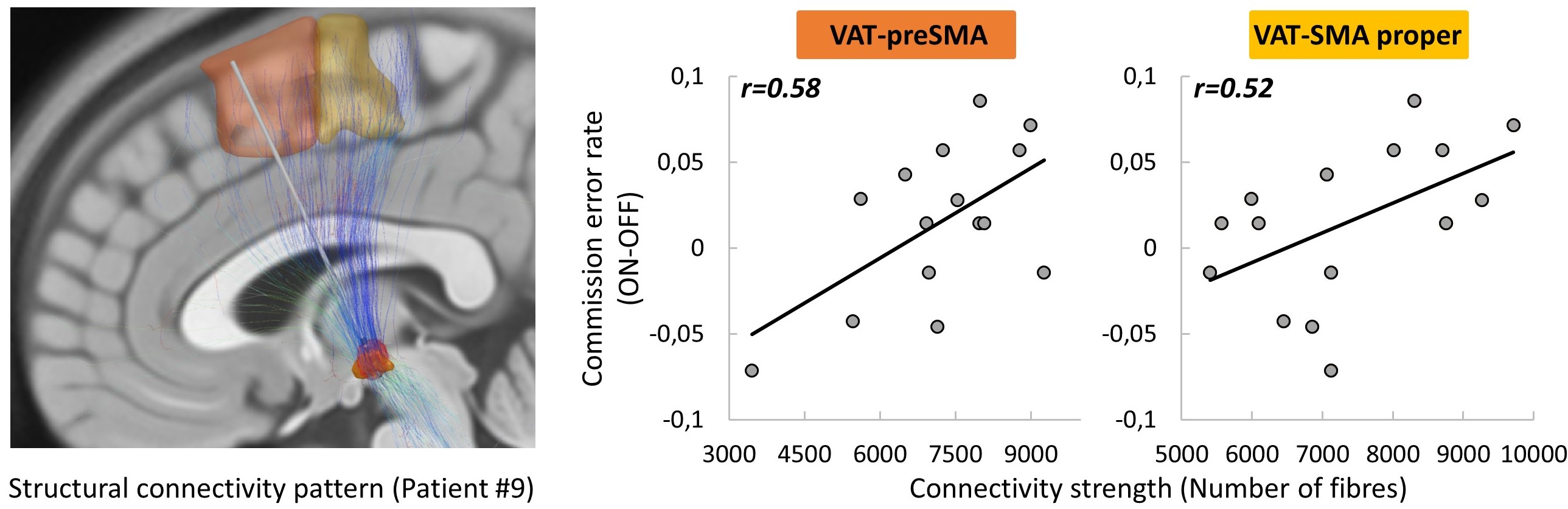
Results: STN-DBS improved movement initiation (reaction time, p=0.003) but impaired response inhibition (commission error rate, p=0.041). The strength of the structural connectivity between the VAT and the SMA was correlated with both the improvement in reaction time (SMA proper: r=.55, p=.032; pre-SMA: r=.68, p=.006) and the worsening of commission error rate (SMA proper: r=.52, p=.048; pre-SMA: r=.58, p=.023).
[Figure 1]
Conclusion: As predicted by the antidromic hypothesis, the larger the structural connectivity between the STN portion activated by DBS and the SMA, the larger the improvement in movement initiation delay and the greater the difficulty in refraining from reacting.
References: [1] Horn, A., Li, N., Dembek, T.A., Kappel, A., Boulay, C., Ewert, S., Tietze, A., Husch, A., Perera, T., Neumann, W.-J., et al. (2019). Lead-DBS v2: Towards a comprehensive pipeline for deep brain stimulation imaging. Neuroimage 184, 293–316. [2] Ewert, S., Plettig, P., Li, N., Chakravarty, M.M., Collins, D.L., Herrington, T.M., Kühn, A.A., and Horn, A. (2018). Toward defining deep brain stimulation targets in MNI space: A subcortical atlas based on multimodal MRI, histology and structural connectivity. Neuroimage 170, 271–282.
To cite this abstract in AMA style:
G. Meyer, M. Albares, S. Thobois, E. Broussolle, G. Polo, P. Baraduc, B. Ballanger, ML. Welter, B. Lau, P. Boulinguez. The volume of STN tissue activated by DBS and the strength of its structural connectivity with SMA predicts the effects of STN-DBS on response inhibition [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/the-volume-of-stn-tissue-activated-by-dbs-and-the-strength-of-its-structural-connectivity-with-sma-predicts-the-effects-of-stn-dbs-on-response-inhibition/. Accessed January 8, 2026.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/the-volume-of-stn-tissue-activated-by-dbs-and-the-strength-of-its-structural-connectivity-with-sma-predicts-the-effects-of-stn-dbs-on-response-inhibition/