Objective: We investigate the functional interaction between two amyloidogenic (amylin and synuclein) proteins as a potential mechanism for neurodegeneration in Parkinson disease (PD).
Background: PD is a progressive neurodegenerative disease but the precise mechanisms inducing neuronal death still remain unknown1. Type 2 diabetes mellitus (T2D) seems to be a risk factor for developing PD. Both PD and T2D, are caused by the abnormal deposition of the aggregated proteins alpha synuclein (α-syn) and amylin, respectively. Our group recently described a-syn aggregates and phosphorylated forms of the tau protein in pancreatic tissue of diabetic, PD and Alzheimer´s disease (AD) patients2,3. Besides these two pathologies share pathological processes the common molecular link remains elusive.
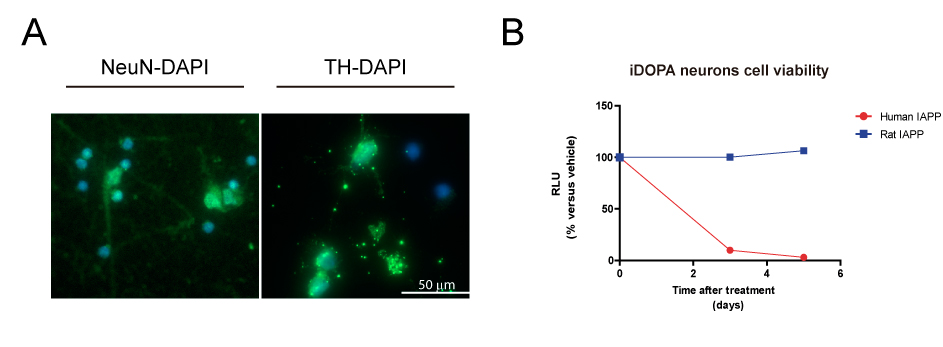
Method: We assessed amylin and pathogenic forms of α-syn levels in the substantia Nigra pars compacta (SNc) of PD and age-matched healthy control brains by quantitative polymerase chain reaction (qPCR) and enzyme-linked immunosorbent assay (ELISA). To elucidate the molecular changes produced by amyloidogenic amylin, we studied the transcriptomic changes of IPSC-derived dopaminergic neurons in response to amylin treatment by RNA sequencing (RNA-seq). These changes in the transcriptome will be further studied in mouse α-syn knock-out mice overexpressing human α-syn (ref) treated with human amylin.
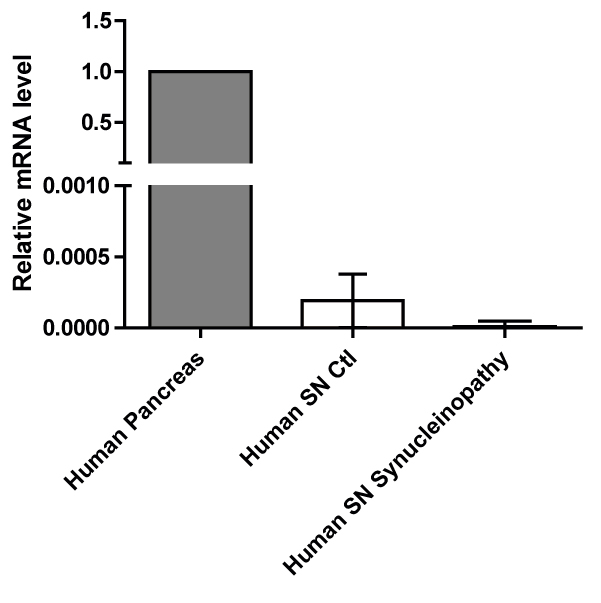
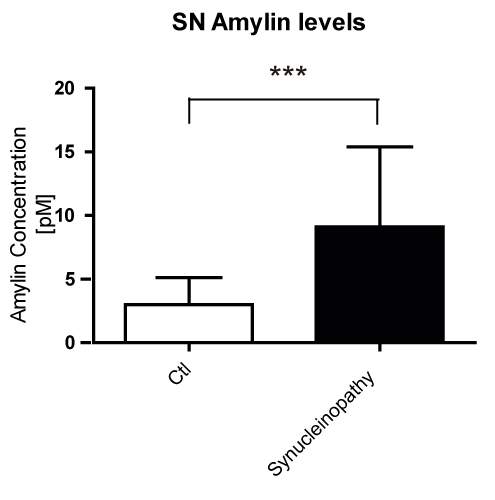
Results: Our results showed a significant increase in amylin and pathogenic α-syn protein levels of PD SN compared to healthy controls. We also showed that amylin gene (IAPP) expression was hardly detectable compared to the IAPP expression levels in human pancreas. Amyloidogenic human amylin significantly reduced the viability of dopaminergic neurons in vitro and elicited alterations in their transcriptomic profile particularly in several key pathways related to neuronal viability. Intra-peritoneal, intraventricular and SN injections of human amylin to mouse α-syn knock-out mice overexpressing human α-syn induced the phosphorylation of serine 129 of the α-syn protein.
Conclusion: Our results indicate that amylin may play an important role in PD neuronal death progression by interacting with endogenous α-syn and prompting the formation of oligomeric forms which will initiate and worsen the neurodegenerative process in the SN.
References: 1. Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Prim. 2017;3:1-21. doi:10.1038/nrdp.2017.13 2. Martinez-Valbuena I, Amat-Villegas I, Valenti-Azcarate R, et al. Interaction of amyloidogenic proteins in pancreatic β cells from subjects with synucleinopathies. Acta Neuropathol. 2018;135(6):877-886. doi:10.1007/s00401-018-1832-0 3. Martinez-Valbuena I, Valenti-Azcarate R, Amat-Villegas I, et al. Amylin as a potential link between type 2 diabetes and alzheimer disease. Ann Neurol. 2019;86(4):539-551. doi:10.1002/ana.25570
To cite this abstract in AMA style:
R. Bugallo, I. Martínez-Valbuena, I. Marcilla, MC. Caballero, A. Vilas-Zornoza, E. Guruceaga, JA. Sánchez-Arias, S. Ursua, A. Pérez-Mediavilla, MR. Luquin. The role of amylin in Parkinson´s disease neurodegenerative process [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/the-role-of-amylin-in-parkinsons-disease-neurodegenerative-process/. Accessed April 2, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/the-role-of-amylin-in-parkinsons-disease-neurodegenerative-process/