Session Information
Date: Saturday, October 6, 2018
Session Title: Cognitive Disorders
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
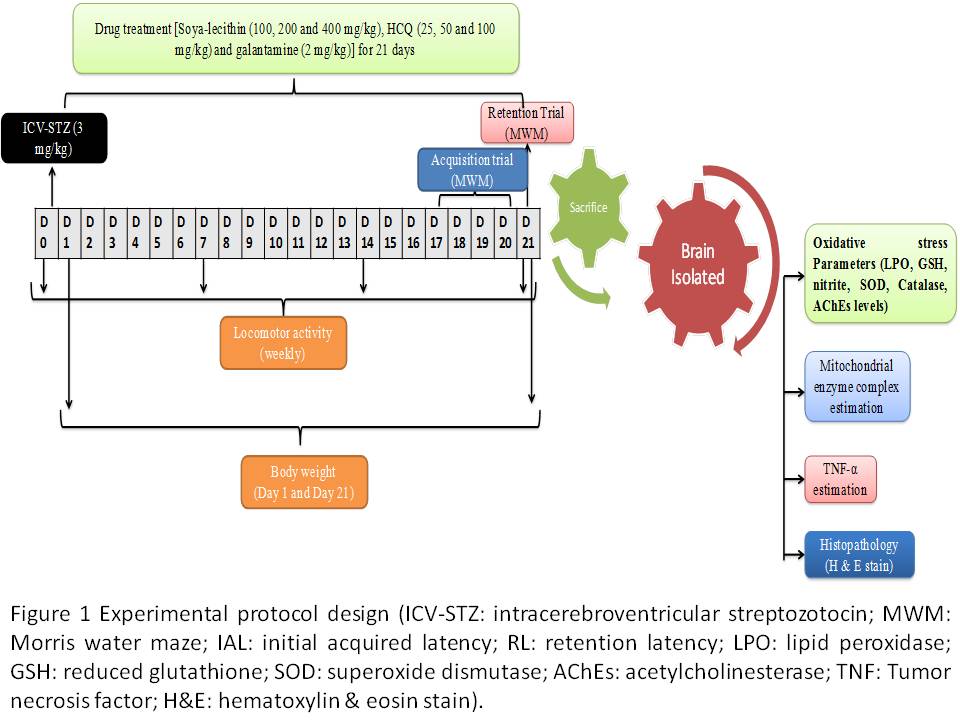
Objective: The present study investigates the possible neuroprotective potential of galantamine with soya-lecithin and HCQ against intracerebroventricular streptozotocin (ICV-STZ) induced memory impairment in a rat model of sporadic dementia of Alzheimer’s type
Background: Galantamine an acetylcholinesterase (AChEs) inhibitor used for the symptomatic treatment of Alzheimer’s disease. Soya-lecithin is a good source of choline improves cognitive performance. Hydroxychloroquine (HCQ) an antimalarial drug with an anti-inflammatory property.
Methods: Animals received single bilateral ICV injections of STZ (3 mg/kg). Drugs galantamine (2 mg/kg), soya-lecithin (100 & 200 mg/kg), HCQ (25 & 50 mg/kg) and their combination was administered for a period of 21 days. Various neurobehavioral parameters, followed by biochemical (oxidative stress markers), AChEs level, molecular (TNF-α level), mitochondrial respiratory enzyme complexes (I-IV), neurotransmitter levels and histopathological (H&E staining) evaluations.
Results: ICV-STZ administration significantly impaired cognitive performance indicated by MWM test, increased oxidative stress (raised lipid peroxidation, nitrite concentration, reduced glutathione, catalase activity), AChEs level, increased TNF-α level, decrease neurotransmitter levels, mitochondrial dysfunction and histopathological alterations as compared to sham treatment. Chronic treatment with galantamine (2 mg/kg), soya-lecithin (100 & 200 mg/kg), HCQ (25 & 50 mg/kg) significantly improved cognitive performance in MWM test, reduced AChEs activity, neuroinflammation, oxidative damage, TNF-α level, restored mitochondrial respiratory enzyme complex (I-IV) activities and histopathological alterations as compared to ICV-STZ treated animals. Further, combinations of soya-lecithin (100 & 200 mg/kg) and HCQ (25 & 50 mg/kg) with galantamine (2 mg/kg) and soya-lecithin (100 & 200 mg/kg) and HCQ (25 & 50 mg/kg) combination suggests the modulation of the neuroprotective potential as compared to their effect alone in ICV-STZ treated animals. Further, the present study suggests the combination potential of soya-lecithin (100 & 200 mg/kg) and HCQ (25 & 50 mg/kg) with galantamine (2 mg/kg) and it was found that galantamine (2 mg/kg) significantly modulate the neuroprotective potential of soya-lecithin (100 & 200 mg/kg) and HCQ (25 & 50 mg/kg) combination in ICV-STZ treated rats as compared to their effect alone.
Conclusions: The present study suggests that co-administration of galantamine with soya-lecithin and HCQ significantly improves cognitive performance in ICV-STZ treated rats as compared to their effect alone.
References: 1. D.S. Auld, T.J. Kornecook, S. Bastianetto, R. Quirion, Alzheimer’s disease and the basal forebrain cholinergic system: relations to β-amyloid peptides, cognition, and treatment strategies. Prog. Neurobiol. 68 (2002) 209-245. 2. E.X. Albuquerque, M. Alkondon, E.F. Pereira, N.G. Castro, A. Schrattenholz, C.T. Barbosa, R. Bonfante-Cabarcas, Y. Aracava, H.M. Eisenberg, A. Maelicke, Properties of neuronal nicotinic acetylcholine receptors: pharmacological characterization and modulation of synaptic function. J. Pharmacol. Exp. Ther. 280 (1997) 1117-1136. 3. F. Amenta, S. Tayebati, Pathways of acetylcholine synthesis, transport and release as targets for treatment of adult-onset cognitive dysfunction. Curr. Med. Chem. 15 (2008) 488-498. 4. R. Anand, K.D. Gill, A.A. Mahdi, Therapeutics of Alzheimer’s disease: Past, present and future. Neuropharmacol. 76 (2014) 27-50. 5. A.S. Association, 2017, Alzheimer’s disease facts and figures. Alzheimer’s Dement. 13 (2017) 325-373. 6. H. Braak, K. Del Tredici, Alzheimer’s disease: pathogenesis and prevention. Alzheimer’s Dement. 8 (2012) 227-233. 7. P.S. Aisen, K.L. Davis, Inflammatory mechanisms in Alzheimer’s disease. Am. J. Psychiatry 151 (1994) 1105-1113. 8. D.J. Bonda, H.P. Lee, H.G. Lee, A.L. Friedlich, G. Perry, X. Zhu, M.A. Smith, Novel therapeutics for Alzheimer’s disease: an update. Curr. Opin. Drug Discov. Devel. 13 (2010) 235. 9. H. Braak, K. Del Tredici, Alzheimer’s disease: pathogenesis and prevention. Alzheimer’s Dement. 8 (2012) 227-233. 10. P. Eikelenboom, C. Bate, W. Van Gool, J. Hoozemans, J. Rozemuller, R. Veerhuis, A. Williams, Neuroinflammation in Alzheimer’s disease and prion disease. Glia 40 (2002) 232-239. 11. P.T. Francis, A.M. Palmer, M. Snape, G.K. Wilcock, The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J. Neurol. Neurosurg. Psychiatry 66 (1999) 137-147. 12. H. Hampel, D. Prvulovic, S. Teipel, F. Jessen, C. Luckhaus, L. Frölich, M.W.Riepe, R.Dodel, T.Leyhe, L.Bertram, The future of Alzheimer’s disease: the next 10 years. Prog. Neurobiol. 95 (2011) 718-728. 13. C. Barnes, J. Meltzer, F. Houston, G. Orr, K. McGann, G. Wenk, Chronic treatment of old rats with donepezil or galantamine: effects on memory, hippocampal plasticity and nicotinic receptors. Neuroscience 99 (2000) 17-23. 14. A. Kumar, A. Prakash, D. Pahwa, Galantamine potentiates the protective effect of rofecoxib and caffeic acid against intrahippocampal Kainic acid-induced cognitive dysfunction in rat. Brain Res. Bull. 85 (2011) 158-168. 15. N.J. Zvaifler, Update in rheumatology—focus on hydroxychloroquine. American J. Med. 85 (1988) 68-71. 16. W.A. Van Gool, H.C. Weinstein, P.K. Scheltens, G.J. Walstra, Effect of hydroxychloroquine on progression of dementia in early Alzheimer’s disease: an 18-month randomised, double-blind, placebo-controlled study. Lancet 358 (2001) 455-460. 17. Y. Wu, T. Wang, Fractionation of crude soybean lecithin with aqueous ethanol. J. American Oil Chemists’ Society 81 (2004) 697-704. 18. A. Bruni, E. Bigon, E. Boarato, L. Mietto, A. Leon, G. Toffano, Interaction between nerve growth factor and lysophosphatidylserine on rat peritoneal mast cells. FEBS letters 138 (1982) 190-192. 19. A. Bruni, G. Toffano, Lysophosphatidylserine, a short-lived intermediate with plasma membrane regulatory properties. Pharmacol. Res. Comm. 14 (1982) 469-484. 20. F. Casamenti, P. Mantovani, L. Amaducci, G. Pepeu, Effect of phosphatidylserine on acetylcholine output from the cerebral cortex of the rat. J. Neurochem. 32 (1979) 529-533. 21. J. Higgins, L. Flicker, Lecithin for dementia and cognitive impairment. The Cochrane Library. (2000) 22. J. Surh, Y.G. Jeong, G.T. Vladisavljević, On the preparation of lecithin-stabilized oil-in-water emulsions by multi-stage premix membrane emulsification. J. Food Eng. 89 (2008) 164-170. 23. A.A. Abdel-Hamid, A.E.-D.L. El-Firgany, Hydroxychloroquine hindering of diabetic isletopathy carries its signature on the inflammatory cytokines. J. Mol. Histol. 47 (2016) 183-193. 24. M. Furushiro, S. Suzuki, Y. Shishido, M. Sakai, H. Yamatoya, S. Kudo, S. Hashimoto, T. Yokokura, Effects of oral administration of soybean lecithin transphosphatidylated phosphatidylserine on impaired learning of passive avoidance in mice. Jpn. J. Pharmacol. 75 (1997) 447-450. 25. J.M. Gutierres, F.B. Carvalho, M.R.C.Schetinger, P.Marisco, P.Agostinho, M.Rodrigues, M.A.Rubin, R.Schmatz, C.R.da Silva, G.D.P. Cognato, Anthocyanins restore behavioral and biochemical changes caused by streptozotocin-induced sporadic dementia of Alzheimer’s type. Life Sci. 96 (2014) 7-17. 26. A. Singh, A. Kumar, Microglial inhibitory mechanism of coenzyme Q10 against Aβ (1-42) induced cognitive dysfunctions: possible behavioral, biochemical, cellular, and histopathological alterations. Front. Pharmacol. 6 (2015) 268. 27. T. Ishrat, M.B. Khan, M.N. Hoda, S. Yousuf, M. Ahmad, M.A. Ansari, A.S. Ahmad, F.Islam, Coenzyme Q10 modulates cognitive impairment against intracerebroventricular injection of streptozotocin in rats. Behav. Brain Res. 171 (2006) 9-16. 28. G. Paxinos, C. Watson, The rat brain in stereotaxic coordinates 2nd edn. Academic Press, New York. Rasoolijazi, H., Joghataie, MT, Roghani, M., & Nobakht, M., 2007. The beneficial effect of (-)-epigallocatechin-3-gallate in an experimental model of Alzheimer’s disease in rat: a behavioral analysis. Iran Biomed. J. 11 (1986) 237-243. 29. A. Kumar, N. Sharma, J. Mishra, H. Kalonia, Synergistical neuroprotection of rofecoxib and statins against malonic acid induced Huntington’s disease like symptoms and related cognitive dysfunction in rats. Eur. J. Pharmacol. 709 (2013) 1-12. 30. R. Morris, Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 11 (1984) 47-60. 31. E. Wills, Mechanisms of lipid peroxide formation in animal tissues, Biochem. J. 99 (1966) 667-676. 32. M. Ellman, A spectrophotometric method for determination of reduced glutathione in tissues, Anal. Biochem. 74 (1959) 214-226. 33. L.C. Green, D.A. Wagner, J. Glogowski, P.L. Skipper, J.S. Wishnok, S.R. Tannenbaum, et al. Analysis of nitrate, nitrite, and [15 N] nitrate in biological fluids, Anal. Biochem. 126 (1982) 131-138. 34. Y. Kono, Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase, Arch. Biochem. Biophys. 186 (1978) 189-195. 35. H. Luck, Catalase. Methods of Enzymatic Analysis, (1965) 885-888. 36. G.L. Ellman, K.D. Courtney, V. Andres, R.M. Featherstone. A new and rapid colorimetric determination of acetylcholinesterase activity, Biochem. Pharmacol. 7 (1961) 88-95. 37. A.G. Gornall, C.J. Bardawill, M.M. David, Determination of serum proteins by means of the biuret reaction, J. Biol. Chem. 177 (1949) 751-766. 38. S.B. Berman, T.G. Hastings, Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria, J. Neurochem. 73 (1999) 1127-1137. 39. T.E. King, R.L. Howard, [52] Preparations and properties of soluble NADH dehydrogenases from cardiac muscle, Method. Enzymol. 10 (1967) 275-294. 40. T.E. King, [58] Preparation of succinate dehydrogenase and reconstitution of succinate oxidase, Method. Enzymol. 10 (1967) 322-331. 41. Y. Liu, D.A. Peterson, H.Kimura, D. Schubert, Mechanism of cellular 3‐(4, 5‐dimethylthiazol‐2‐yl)‐2, 5‐diphenyltetrazolium bromide (MTT) reduction, J. Neurochem. 69 (1997) 581-593. 42. G. Sotocassa, E. Bokuylenstiema, L. Ernster, A. Bergstrand, An electron transport system associated with the outer membrane of liver mitochondria, J. Cell. Biol. 32 (1967) 415-438. 43. A.Prakash, A.Kumar, Role of nuclear receptor on regulation of BDNF and neuroinflammation in hippocampus of β-amyloid animal model of Alzheimer’s disease. Neurotox. Res. 25 (2014b) 335-347. 44. N. Rajasekar, S. Dwivedi, C.Nath, K.Hanif, R.Shukla, Protection of streptozotocin induced insulin receptor dysfunction, neuroinflammation and amyloidogenesis in astrocytes by insulin. Neuropharmacol. 86 (2014) 337-352. 45. K. Reeta, D.Singh, Y.Gupta, Chronic treatment with taurine after intracerebroventricular streptozotocin injection improves cognitive dysfunction in rats by modulating oxidative stress, cholinergic functions and neuroinflammation. Neurochem. Int. 108 (2017) 146-156. 46. M. Sharma, Y. Gupta, Effect of alpha lipoic acid on intracerebroventricular streptozotocin model of cognitive impairment in rats. Eur. Neuropsychopharmacol. 13 (2003) 241-247.
To cite this abstract in AMA style:
A. Singh, A. Kumar. The possible neuroprotective potential of galantamine along with soya-lecithin and hydroxychloroquine against ICV-STZ induced cognitive dysfunction in rats [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/the-possible-neuroprotective-potential-of-galantamine-along-with-soya-lecithin-and-hydroxychloroquine-against-icv-stz-induced-cognitive-dysfunction-in-rats/. Accessed December 23, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/the-possible-neuroprotective-potential-of-galantamine-along-with-soya-lecithin-and-hydroxychloroquine-against-icv-stz-induced-cognitive-dysfunction-in-rats/