Category: Parkinson’s Disease: Clinical Trials
Objective: To evaluate if treatment with opicapone (OPC) in patients with Parkinson’s disease (PD) improves pain related to end-of-dose fluctuations, consequently improving patients’ well-being.
Background: OPC proved to be effective in the treatment of end-of-dose motor fluctuations in patients with PD [1,2]. End-of-dose motor fluctuations and associated pain are commonly observed in patients with PD receiving L-dopa/DOPA decarboxylase inhibitors (DDCIs). They have a detrimental impact on the quality of life [3] and are partly mediated via dopaminergic pathways [4].
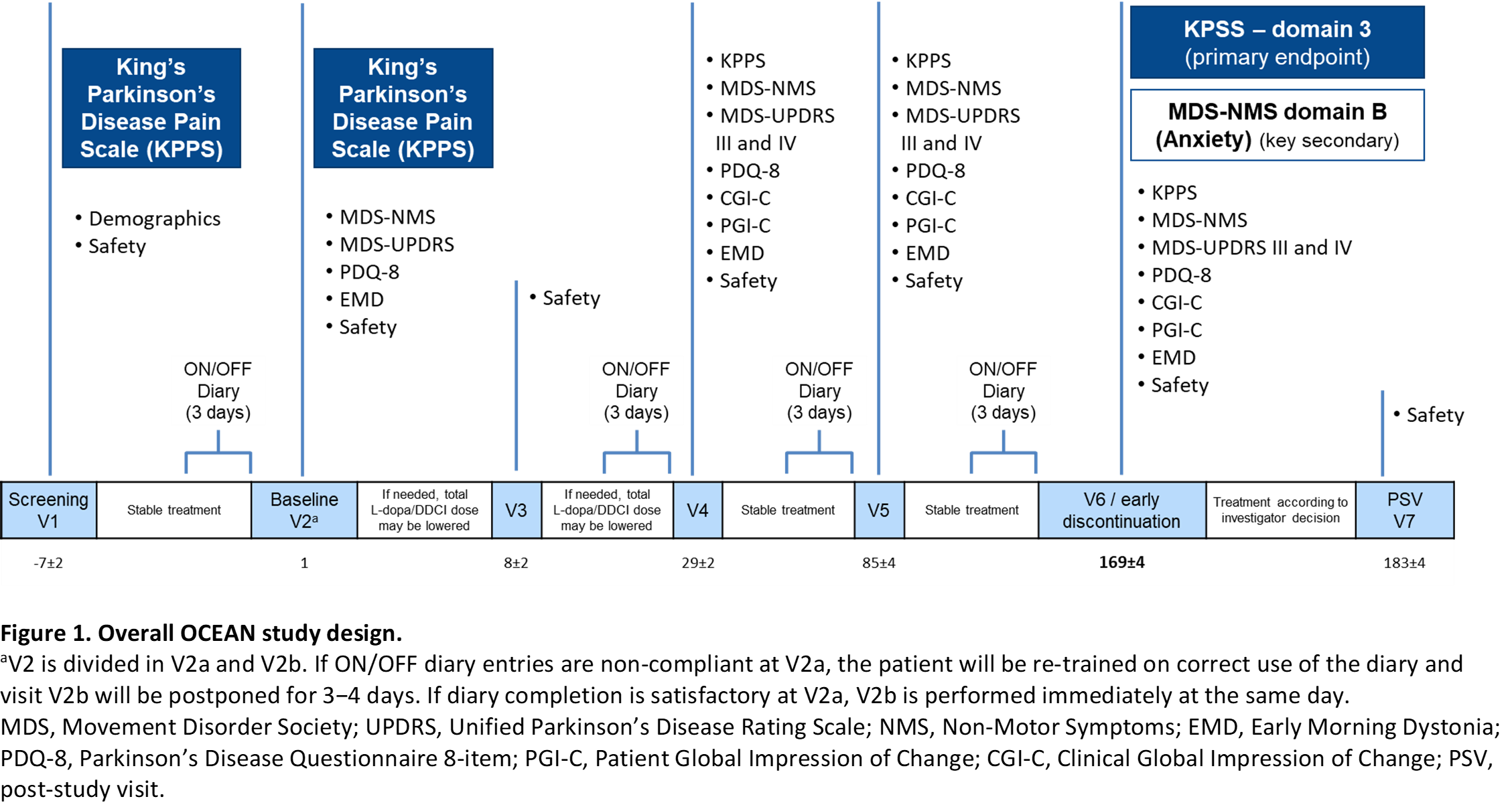
Method: Patients (≥30 years old) with idiopathic PD, who were treated with 3 to 8 daily oral doses of L-dopa/DDCI and experienced pain associated with ‘wearing-off’ (end-of-dose deterioration), will be randomized (1:1) to OPC 50 mg once-daily or placebo during a 24-week evaluation period (Figure 1). To detect a minimum clinically-relevant magnitude of effect between arms, 70 patients per group are necessary.
Results: The primary endpoint is change from baseline in domain 3 (fluctuation-related pain) of the King’s-Parkinson’s-Disease-Pain-Scale (KPPS). Secondary endpoints include tolerability, functional motor and non-motor assessments (KPSS, MDS-Non-Motor Symptoms [NMS], Parkinson’s Disease Questionnaire-8 [PDQ-8], Hauser’s home diary), and global impression of change scales (Clinical Global Impression of Change [CGI-C], Patient Global Impression of Change [PGI-C]). Study sites are in Germany, Italy, Portugal, Spain, and the UK. First-patient-in is expected for 2021 and last-patient-out for late 2022. Timelines might be impacted by the COVID-19 pandemic situation.
Conclusion: This study will further evaluate the impact of 50 mg opicapone once daily as adjunctive therapy to L-dopa/DDCI on fluctuation-associated pain.
References: 1. Ferreira et al., Lancet Neurol. 2016;15(2):154-165 2. Lees et al., JAMA Neurol. 2017;74(2):197-206 3. Gökçal et al., Noro Psikiyatr Ars. 2017;54(2):143-148 4. Antonini et al., Eur J Neurol. 2018;25(7):917-e69
To cite this abstract in AMA style:
K. Chaudhuri, P. Odin, J. Ferreira, A. Antonini, O. Rascol, M. Kurtis, A. Storch, K. Bannister, R. Costa, D. Magalhães, J. Rocha, P. Soares-da-Silva. The OCEAN (OpiCapone Effect on motor fluctuations and associated pAiN) study in Parkinson’s disease: design and rationale of a randomized double-blind placebo-controlled trial [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/the-ocean-opicapone-effect-on-motor-fluctuations-and-associated-pain-study-in-parkinsons-disease-design-and-rationale-of-a-randomized-double-blind-placebo-controlled-trial/. Accessed April 22, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/the-ocean-opicapone-effect-on-motor-fluctuations-and-associated-pain-study-in-parkinsons-disease-design-and-rationale-of-a-randomized-double-blind-placebo-controlled-trial/