Category: Rare Genetic and Metabolic Diseases
Objective: To establish an innovative process for fast and efficient pathways of delivering gene-based therapy for ultra-rare neurological disorders from the lab to the clinic
Background: Nearly 7,000 rare diseases affect 4-6% of the world’s population and approximately 80% of rare diseases have an identified genetic origin, which serves as a potential target for the emerging field of gene-targeted treatments. Majority of movement disorders are classified as rare diseases.
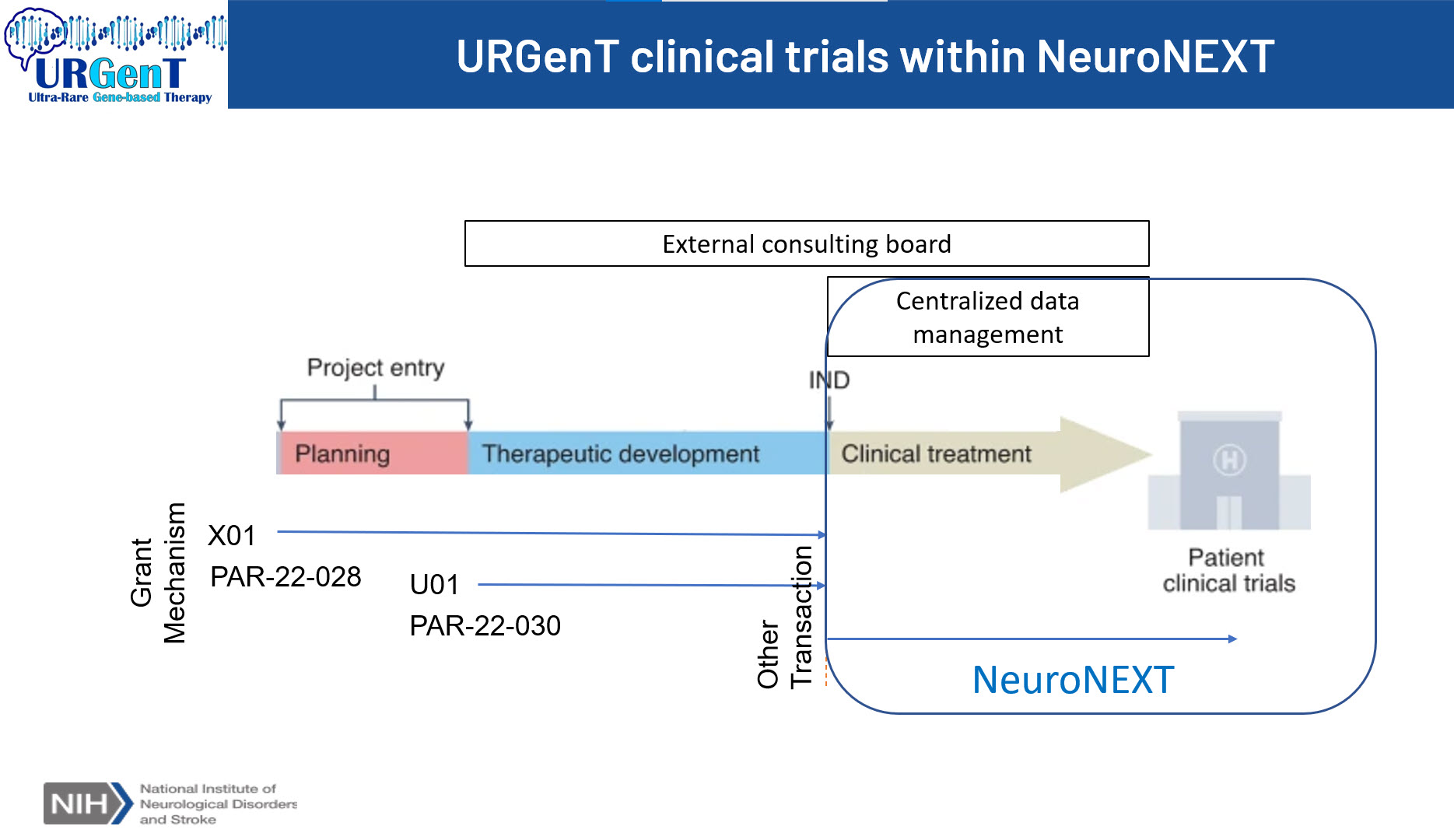
Method: The Ultra-Rare Gene-based Therapy (URGenT) network was launched by the National Institute of Neurological Disorders and Stroke (NINDS) in 2021 to support the late-stage pre-clinical development of gene-based therapies for ultra-rare neurological diseases. Building on this program, NINDS recently expanded the network to include clinical trial resources through the Network for Excellence in Neuroscience Clinical Trials (NeuroNEXT). To facilitate a seamless transition of promising assets from the pre-clinical to the clinical space, NeuroNEXT established the Gene Therapy Consortium to provide guidance on potential challenges that may be unique for gene-based therapy trials.
Results: The URGenT network established R&D contracts and consultants to deliver assistance at different stages of the awarded projects. There are currently four active pre-clinical projects developing gene-based therapies for Menkes disease, Prion disease, ALS, and Aspartylglucosaminuria. NINDS published a Research Opportunity Announcements (ROA) to invite applications for URGenT clinical trials. The URGenT clinical trials program will not be limited to assets developed through the URGenT pre-clinical program; applicants who are not part of the URGenT pre-clinical program may apply to this ROA.
Conclusion: The proposed NINDS model for a seamless transition from preclinical to clinical research aims to accelerate the development of gene-based therapeutics for ultra-rare diseases. Standardizing data and resource sharing across diseases will enhance the efficiency and accessibility of therapy development for ultra-rare diseases. Throughout the implementation of the URGenT network, NINDS will actively engage with multiple stakeholders. This collaboration will to pave a path to deliver novel therapeutics reliably, sustainably, and quickly to patients with movement disorders caused by ultra-rare neurological disorders.
NeuroNEXT infrastructure
URGenT clinical trials within NeuroNEXT
References: Jinnah HA, Albanese A, Bhatia KP, et al. Treatable inherited rare movement disorders. Mov Disord. 2018;33(1):21-35. doi:10.1002/mds.27140
Schor, N.F., Tamiz, A.P., Koroshetz, W.J. et al. NINDS launches network to develop treatments for ultra-rare neurological diseases. Nat Biotechnol 39, 1497–1499 (2021). https://doi.org/10.1038/s41587-021-01125-w
To cite this abstract in AMA style:
HJ. Cho, A. Videnovic, M. Cudkowicz, C. Coffey, D. Klements, M. Chase, J. Ohayon, C. Boshoff, A. Tamiz, C. Wright. The NINDS Approach to Therapeutic Development for Ultra-Rare Neurological Disorders, Bridging Translational and Clinical Frontiers in Gene-based Therapy [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/the-ninds-approach-to-therapeutic-development-for-ultra-rare-neurological-disorders-bridging-translational-and-clinical-frontiers-in-gene-based-therapy/. Accessed April 18, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/the-ninds-approach-to-therapeutic-development-for-ultra-rare-neurological-disorders-bridging-translational-and-clinical-frontiers-in-gene-based-therapy/