Session Information
Date: Monday, October 8, 2018
Session Title: Rating Scales
Session Time: 1:15pm-2:45pm
Location: Hall 3FG
Objective: To provide an update on the ongoing international validation programme of an expanded, refined and improved scale for the assessment of non-motor symptoms (NMS) in Parkinson’s disease (PD), the MDS-NMS, sponsored by the International Parkinson and Movement Disorders Society (IPMDS).
Background: The PD NMS scale (NMSS) was developed in 2006 and remains the only dedicated composite scale to assess NMS in PD. As it needed updating, a project to revise and update the NMSS, now called the MDS-NMS, was commissioned by the IPMDS. The MDS-NMS has already undergone cognitive pre-testing and a final version of MDS-NMS was developed. It includes 13 domains with 52 items total, each item scoring for frequency (0 to 4) and severity (0 to 4), which are multiplied to obtain the item score (0 to 16). The domain scores result from sum of the item scores within each domain. Additionally, the scale includes an optional section for evaluating non-motor fluctuations (NMFs) for 8 domains.
Methods: In this cross-sectional, open, multicentre international validation study, we report clinical data from administration of the MDS-NMS. Acceptability, internal consistency, reliability, construct validity and precision in 400 non-demented PD patients (MoCA score>20) is ongoing. Test-retest reliability, assessed after two weeks (average) as well as inter-rater reliability are reported in 100 patients. NMS are also assessed with existing measures, as are motor symptoms and global severity of PD.
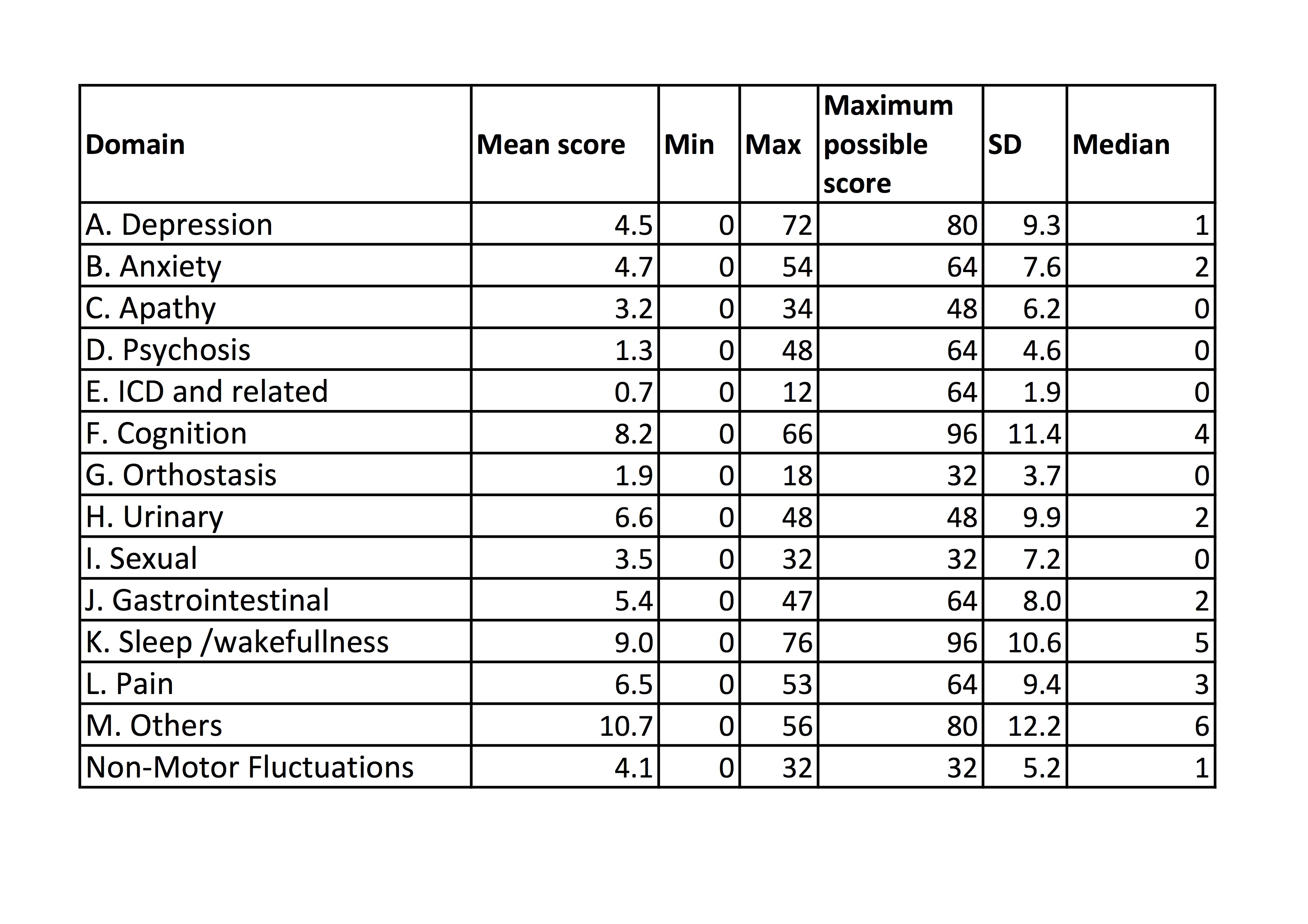
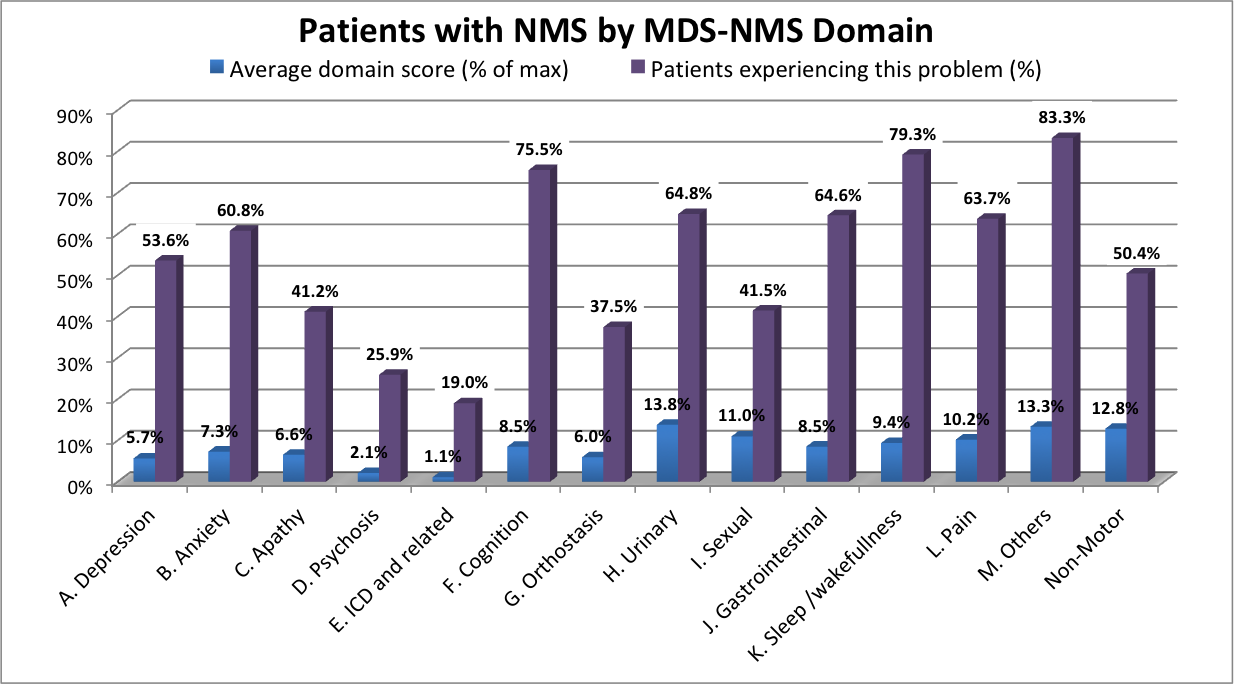
Results: 347 patients, 58.8% male, mean age 68.7 yrs (35-93), median H&Y 2 (1-5), mean duration of disease 7.2 yrs (0-30). Mean and median scores for the 13 domains and for NMFs for 8 domains are listed [Table 1]. Standardised domain scores (% of maximum possible score), and % of patients experiencing any problems in each domain are illustrated [Figure 1]. The most frequently reported problems were found in domains “Others” (including loss of sense of smell) (83.3%) “Sleep/wakefulness” (79.3%), “Cognition” (75.5%), and “Urinary” (64.8%). The greatest average severity of symptoms were found in “Urinary” (mean 13.8%), “Others” (13.3%), “Sexual” (12.9%) and “Pain” (10.2%).
Conclusions: Interim results suggest the MDS-NMS may perform well as an outcome measure in clinical trials and epidemiological studies to assess the wide range of NMS that occur in PD.
To cite this abstract in AMA style:
K. Ray Chaudhuri, D. Weintraub, A. Rizos, A. Schrag, P. Martinez-Martin. The International Parkinson and Movement Disorder Society Non-Motor Scale (MDS-NMS) for Parkinson’s Disease: Preliminary results from an international validation [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/the-international-parkinson-and-movement-disorder-society-non-motor-scale-mds-nms-for-parkinsons-disease-preliminary-results-from-an-international-validation/. Accessed February 3, 2026.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/the-international-parkinson-and-movement-disorder-society-non-motor-scale-mds-nms-for-parkinsons-disease-preliminary-results-from-an-international-validation/