Session Information
Date: Sunday, October 7, 2018
Session Title: Technology
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
Objective: Open-label, multi-center pilot study (PD0049) to evaluate the impact of Kinesia 360 (an “at home” wearable device) on motor symptom management in Parkinson’s disease (PD) patients (pts) starting transdermal rotigotine (RTG) treatment.
Background: Objective feedback from wearable devices may help clinicians more accurately assess motor function and customize RTG dosage, which may help improve motor symptoms. Kinesia ONE measures specific motor tasks via a sensor on the index finger. Kinesia 360 provides continuous motor symptom monitoring via wrist and ankle sensors.
Methods: Adults with PD and insufficiently controlled motor symptoms prescribed RTG at a clinic visit were randomized 1:1 to Control Group (CG) or Experimental Group (EG) before starting RTG. Motor symptoms were assessed in all pts at baseline (BL) and Week 12 (W12) using Unified PD Rating Scale (UPDRS) III and Kinesia ONE. Between BL and W12, EG used Kinesia 360 at home; their physicians used Kinesia 360 data to supplement standard clinical care for RTG dosage adjustment decisions. Efficacy variables: change from BL to W12 in UPDRS II and III scores and Kinesia ONE variables, W12 RTG dosage, RTG dosage changes between BL and W12, RTG discontinuation. Safety variable: adverse events (AEs).
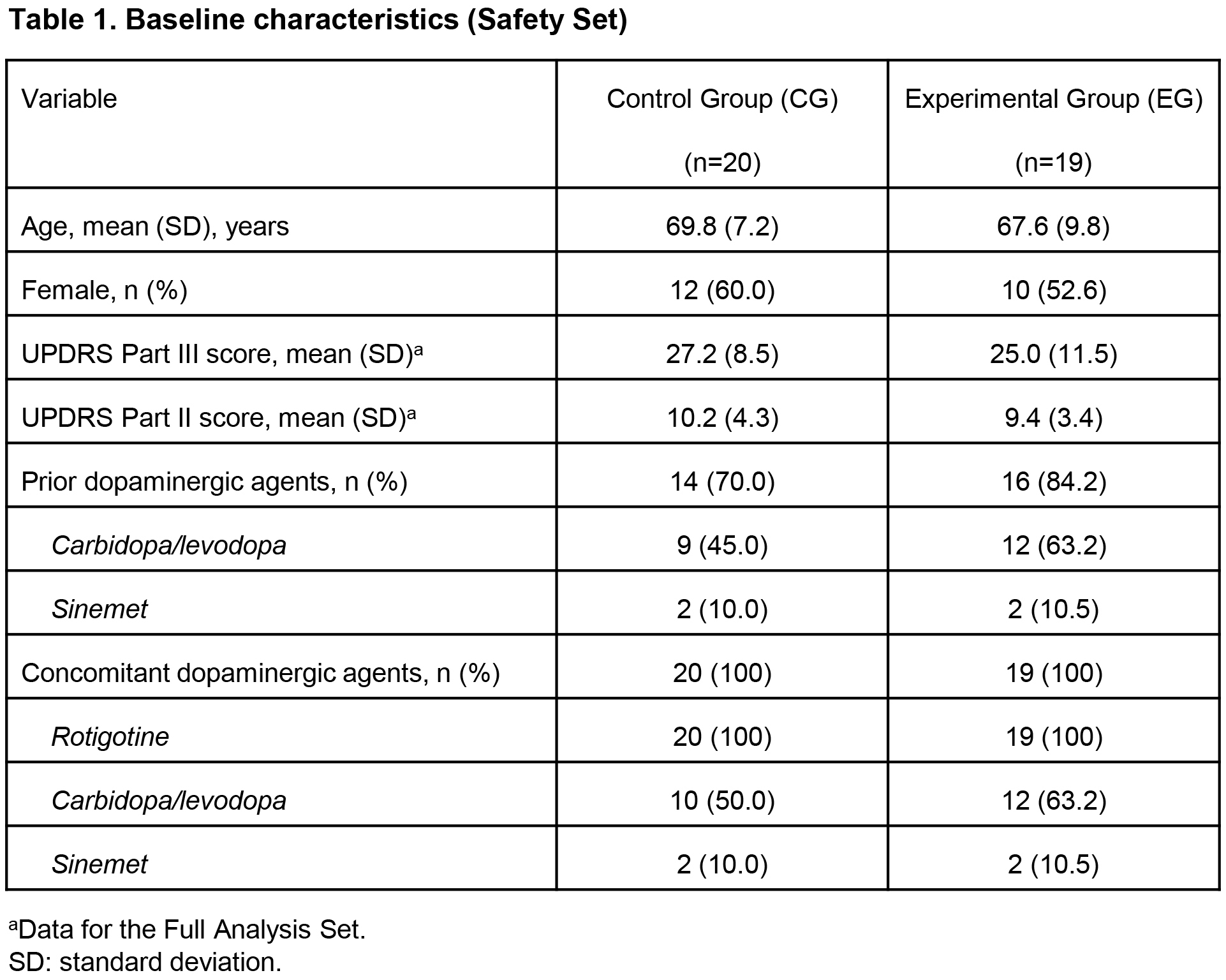
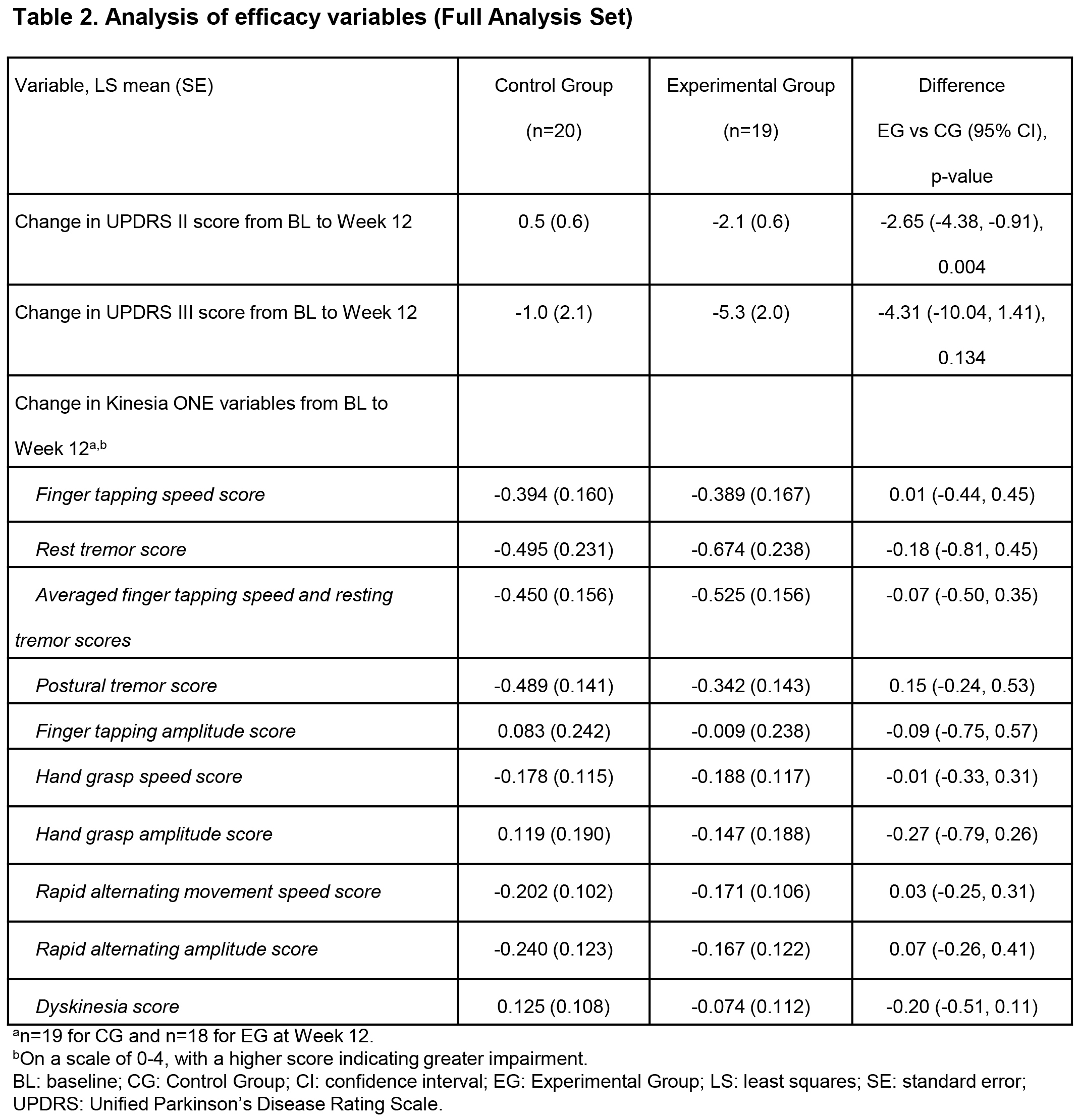
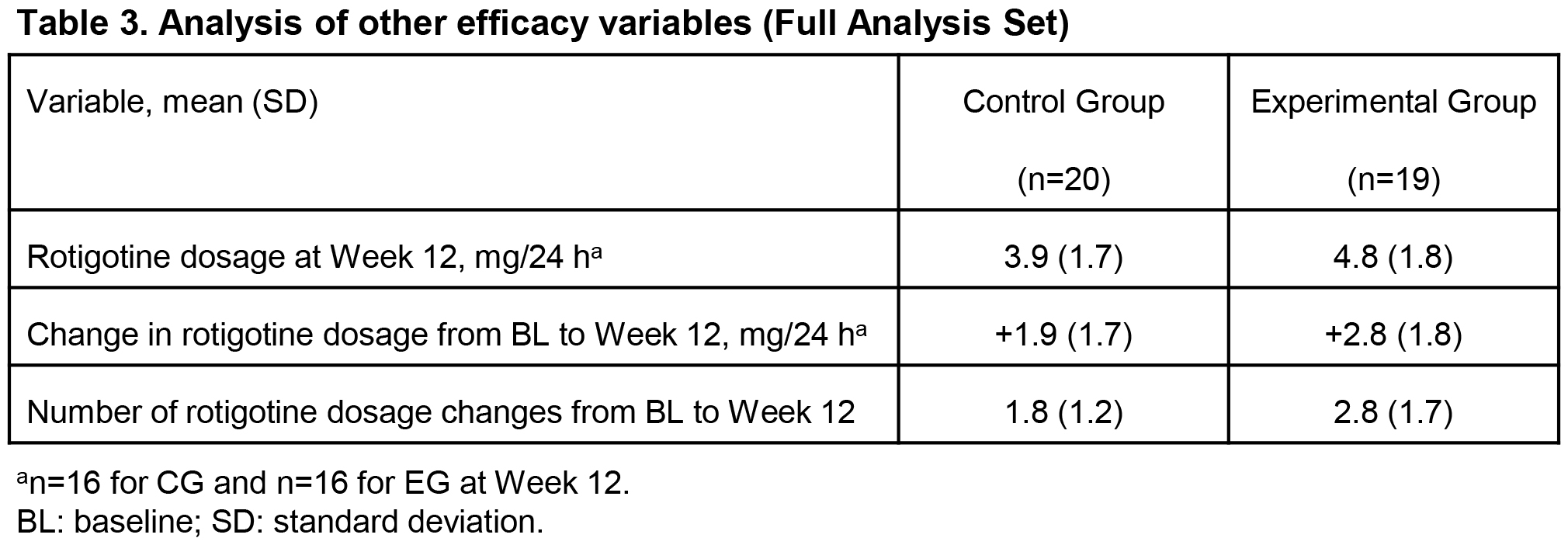
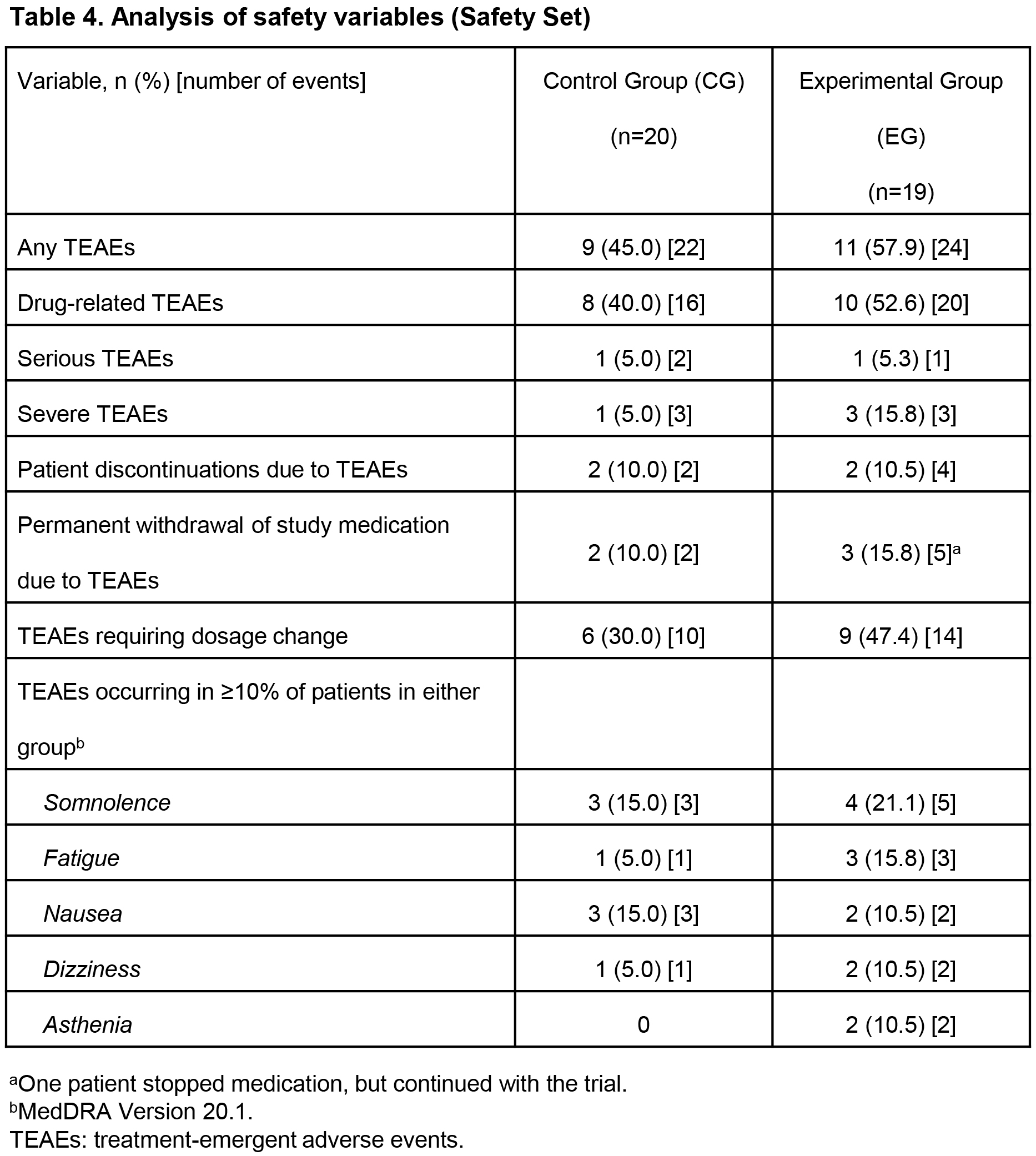
Results: At W12, least squares (LS) mean improvements in UPDRS II (-2.1 vs 0.5, p=0.004) and UPDRS III (-5.3 vs -1.0, p=0.134) were clinically meaningfully greater, and mean RTG dosage higher (4.8 vs 3.9 mg/24 h) in EG (n=19) vs CG (n=20). [table1] [table2] [table3] Mean RTG dosage increase (+2.8 vs +1.9 mg/24 h) and mean number of RTG dosage changes (2.8 vs 1.8) during the study were higher in EG vs CG. [table3] Safety profiles and retention rates were similar. [table4]
Conclusions: In the group using “at home” monitoring, there were more RTG dosage changes and higher mean RTG dosages at W12, probably reflecting closer evaluation of these pts, leading to greater improvement in UPDRS scores; this exploratory study was not statistically powered to detect differences, so these improvements are encouraging. AE profiles in both groups were similar and consistent with the known RTG safety profile. This suggests using a wearable biosensor at home for continuous objective motor symptom monitoring in combination with standard of care enhances clinical decision-making and may improve clinical outcomes of PD pts newly started on RTG.
References: Funded by UCB Pharma.
To cite this abstract in AMA style:
S. Isaacson, B. Boroojerdi, K. Klos, S. Carson, M. Markowitz, D. Heldman, M. Phillips, D. Terricabras, F. Woltering, D. Truong. The impact of a wearable device on Parkinson’s disease motor symptom management in patients starting rotigotine transdermal patch [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/the-impact-of-a-wearable-device-on-parkinsons-disease-motor-symptom-management-in-patients-starting-rotigotine-transdermal-patch/. Accessed October 22, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/the-impact-of-a-wearable-device-on-parkinsons-disease-motor-symptom-management-in-patients-starting-rotigotine-transdermal-patch/