Category: Neuroimaging (Non-PD)
Objective: This study aimed to identify differences in FDG uptake and metabolic covariant network (independent component analysis) in progressive supranuclear palsy (PSP) in comparison with healthy controls.
Background: FDG-PET/CT imaging is a promising tool for identifying specific biomarkers in PSP, which could aid in differential diagnosis and explore the metabolic pattern in PSP. Source-based morphometry (SBM), investigating the metabolic covariant network was rarely applied in PSP, which could provide additional insight into the understanding in pathophysiological mechanisms.
Method: FDG PET/CT data were collected from 72 PSP patients and 70 age- and sex-matched healthy controls. PSP patients received a battery of neuropsychological assessments and PSP rating scale (PSPRS). Intensity normalization was performed using the histogram method. Voxel-based differences between patients and controls were compared using independent t-tests. To measure differences in the source-based morphometry (SBM), the weights of the mixing matrix were extracted and corrected for clinical parameters using a generalized linear model.
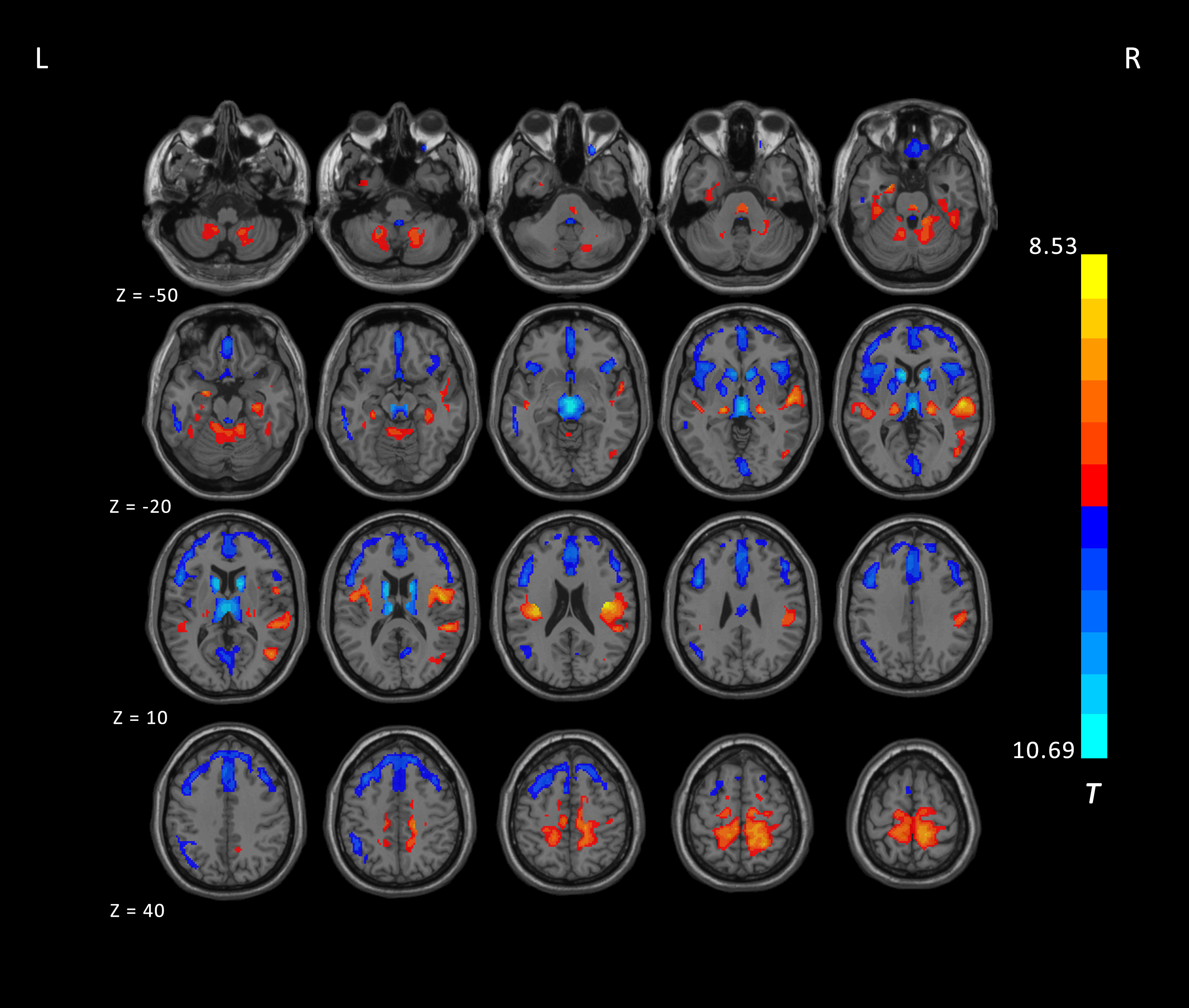
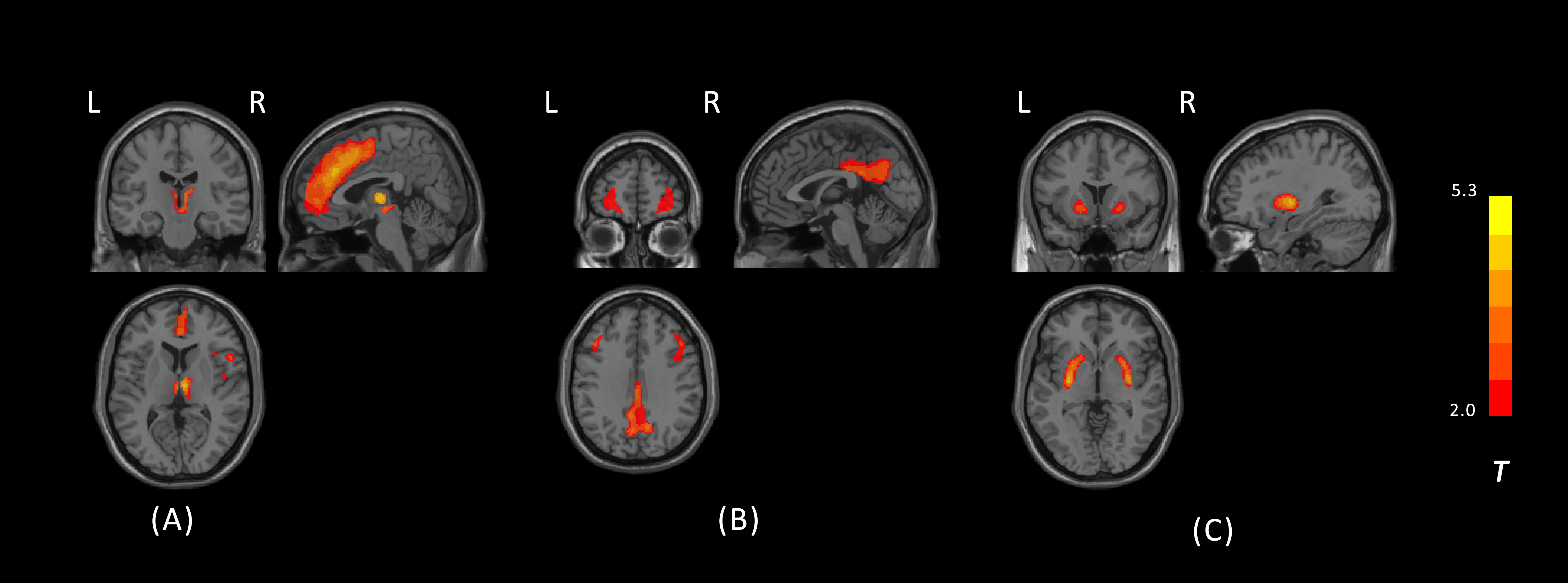
Results: Independent t-tests revealed significant differences in FDG uptake between PSP patients and controls (corrected by GRF, voxel level p < 0.001, cluster level p <0.05; Fig. 1). PSP patients showed decreased uptake in several regions, including the prefrontal lobe, anterior cingulate gyrus, basal ganglia, thalamus, cerebellum, and midbrain, as well as increased uptake in the parietal and temporal lobes. In the SBM analysis, ten components were examined for both patients and controls, and three independent components existed in PSP patients but not in controls (Fig. 2). The component representing the anterior cingulate with thalamus was negatively correlated with disease duration (p=0.02, r= -2.39), PSPRS (p=0.0005, r= -3.74), and PSPRS-gait and midline sub-score (p=0.004, r= -3.01). The component representing the basal ganglia was also negatively correlated with disease duration (p=0.004, r= -3.01). The component representing the posterior cingulate gyrus, parietal lobe, and prefrontal lobe was negatively correlated with MMSE (p=0.008, r= 2.75) and positively correlated with PSPRS-mentation sub-score (p=0.005, r= -2.94).
Conclusion: The hypometabolism in anterior cingulate gyrus and thalamus might be potential biomarkers of disease progression.
To cite this abstract in AMA style:
B. Wang, H. Wang. The hypometabolism in anterior cingulate gyrus and thalamus: potential biomarkers of disease progression in progressive supranuclear palsy [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/the-hypometabolism-in-anterior-cingulate-gyrus-and-thalamus-potential-biomarkers-of-disease-progression-in-progressive-supranuclear-palsy/. Accessed April 20, 2025.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/the-hypometabolism-in-anterior-cingulate-gyrus-and-thalamus-potential-biomarkers-of-disease-progression-in-progressive-supranuclear-palsy/