Category: Parkinson’s Disease: Clinical Trials
Objective: This study aims to explore the potential of opicapone (OPC) to enhance the clinical benefit of levodopa (L-dopa)/dopa decarboxylase inhibitor (DDCi) in patients with early-stage Parkinson’s disease (PD) on stable treatment.
Background: OPC proved to be effective in the treatment of end-of-dose motor fluctuations in patients with PD [1,2]. When OPC is co-administered with L-dopa and DDCis, peripheral catechol-O-methyltransferase (COMT) is inhibited increasing L-dopa bioavailability.
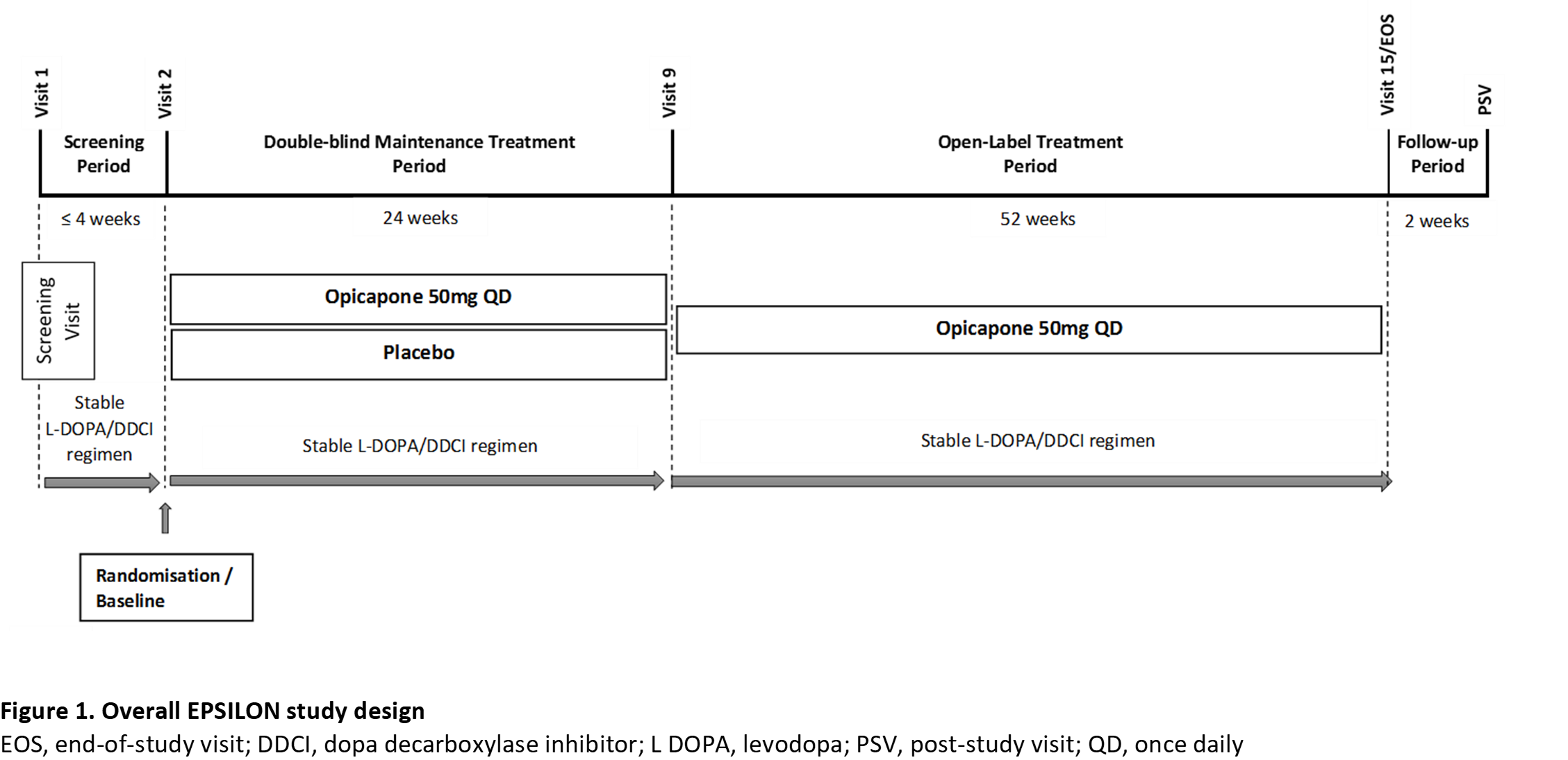
Method: Patients (aged 30−80 years) with idiopathic PD, treated with 3-4 daily oral doses of up to 500 mg L-dopa, with signs of treatable motor disability but no motor complications will be randomized in a 1:1 ratio to receive OPC 50 mg once-daily or placebo during a 6-month double-blind evaluation period. The patients’ L-dopa/DDCi regimen should remain stable throughout the double-blind period. At the end of the double-blind period, patients may enter an additional 1-year, open-label period of OPC 50 mg treatment (Figure 1). 162 patients in each group are necessary to detect a minimum clinically-relevant magnitude of effect between arms.
Results: Change from baseline in the MDS-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part III is the primary endpoint. Secondary endpoints include tolerability, functional motor and non-motor assessments (MDS-UPDRS, Non-Motor Symptoms Scale [NMSS], Parkinson’s Disease Questionnaire-39 [PDQ-39], Parkinson’s Disease Sleep Scale-2 [PDSS-2]), and global impression of change scales (Clinical Global Impression of Change [CGI-C], Patient Global Impression of Change [PGI-C]). First-patient-in is expected for early 2021 and last-patient-out from double-blind period in late 2022. Timelines might be impacted by the COVID-19 pandemic situation.
Conclusion: This study will evaluate the efficacy of once-daily OPC 50 mg as add-on to stable L-dopa/DDCi therapy in patients with early-stage PD.
References: 1. Ferreira et al., Lancet Neurol. 2016;15(2):154-165; 2. Lees et al., JAMA Neurol. 2017;74(2):197-206
To cite this abstract in AMA style:
J. Ferreira, W. Poewe, O. Rascol, F. Stocchi, A. Antonini, J. Moreira, J. Rocha, P. Soares-da-Silva. The EPSILON (Early ParkinSon wIth L-dopa/DDCi and OpicapoNe) study in early Parkinson’s disease: design and rationale of a phase III, double-blind, randomized, placebo-controlled study [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/the-epsilon-early-parkinson-with-l-dopa-ddci-and-opicapone-study-in-early-parkinsons-disease-design-and-rationale-of-a-phase-iii-double-blind-randomized-placebo-controlled-study/. Accessed December 29, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/the-epsilon-early-parkinson-with-l-dopa-ddci-and-opicapone-study-in-early-parkinsons-disease-design-and-rationale-of-a-phase-iii-double-blind-randomized-placebo-controlled-study/