Objective: In this double blind, sham controlled study on 30 advanced Parkinson’s disease (aPD) patients we used inertial motion sensors (IMS) to characterize the effect on transcutaneous auricular vagal nerve stimulation (taVNS) on gait and turning.
Background: Studies have shown that taVNS has the potential to access diffuse neuromodulatory networks (DNN) through afferent vagal connections with the brainstem nuclei, including nucl. Rahpe and locus coeruleus [1]. DNN dysfunction contributes to abnormal gait in aPD. Preliminary data on both animal models and patients suggests taVNS may improve gait in PD [2 – 7].
Method: We included 30 PD patients (HY>=2), each completed 1 visit during which they performed instrumented stand and walk tests and double 360º turns while receiving taVNS at 25Hz (taVNS25), taVNS at 100Hz (taVNS100) and earlobe stimulation at 25Hz (sVNS). They were encouraged to repeat both tests while counting. They were equipped with 6 IMS and we used the provided sensor software to analyse Arm Swing Velocity, Arm Range of Motion (RoM), Stride Length, Gait Speed, arm RoM Asymmetry, Anticipatory Postural Adjustment (APA) duration, First step Duration, and First Step RoM. We also analysed the duration of the first turn, the complete double turn duration and the average number of steps per turn with custom-build algorithms. GLMM were built where the stimulation type was used as a fixed effect and the order of stimulation, previous stimulation and counting were added when appropriate. Outliers were discarded. We computed the effect of VNS on gait measures per condition (estimated marginal means of linear trends) and calculated pairwise comparisons using the Benjamini Hochberg correction.
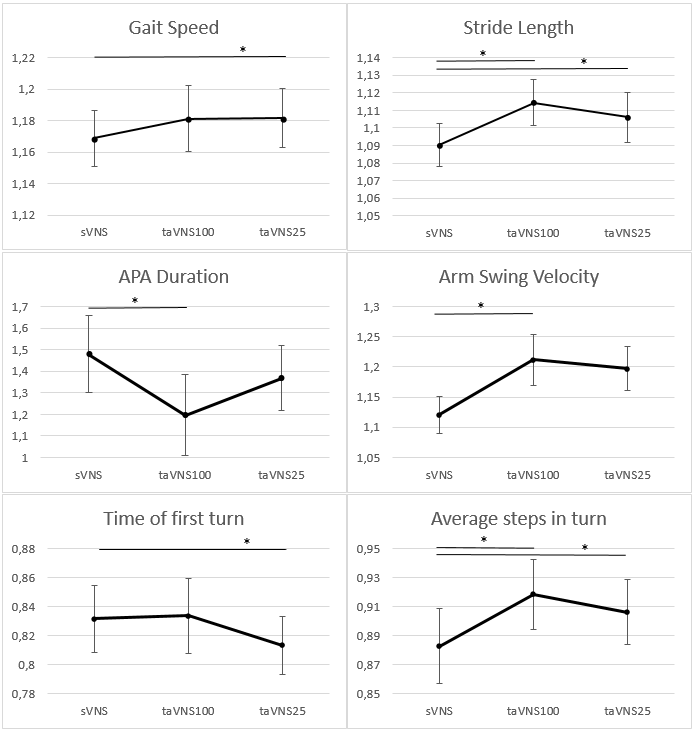
Results: During taVNS100 compared to sVNS arm swing velocity (p = 0.030) and stride length (p = 0.027) and steps per turn (p=0.031) increased, and APA duration decreased (p=0.050). During taVNS25 compared to sVNS stride length (p=0.024), gait speed (p=0.021) and steps per turn (p=0.001) increased and first turn duration decreased (p=0,006) [figure1].There was no effect of stimulation on other analysed measures.
Conclusion: We have found that taVNS has a frequency-specific propensity to improve stride length, arm swing velocity, gait speed and duration of the first turn in aPD patients.
References: [1] Sclocco, Roberta, Ronald G. Garcia, Norman W. Kettner, Harrison P. Fisher, Kylie Isenburg, Maya Makarovsky, Jessica A. Stowell, Jill Goldstein, Riccardo Barbieri, and Vitaly Napadow. 2020. “Stimulus Frequency Modulates Brainstem Response to Respiratory-Gated Transcutaneous Auricular Vagus Nerve Stimulation.” Brain Stimulation 13 (4): 970–78. https://doi.org/10.1016/j.brs.2020.03.011.
[2] Farrand, Ariana Q., Kristi L. Helke, Rebecca A. Gregory, Monika Gooz, Vanessa K. Hinson, and Heather A. Boger. 2017. “Vagus Nerve Stimulation Improves Locomotion and Neuronal Populations in a Model of Parkinson’s Disease.” Brain Stimulation 10 (6): 1045–54. https://doi.org/10.1016/j.brs.2017.08.008.
[3] Jiang, Ying, Zhentang Cao, Huizi Ma, Guihong Wang, Xuemei Wang, Zhan Wang, Yaqin Yang, et al. 2018. “Auricular Vagus Nerve Stimulation Exerts Antiinflammatory Effects and Immune Regulatory Function in a 6-OHDA Model of Parkinson’s Disease.” Neurochemical Research 43 (11): 2155–64. https://doi.org/10.1007/s11064-018-2639-z.
[4] Marano, Massimo, Gaia Anzini, Gabriella Musumeci, Alessandro Magliozzi, Valeria Pozzilli, Fioravante Capone, and Vincenzo Di Lazzaro. 2022. “Transcutaneous Auricular Vagus Stimulation Improves Gait and Reaction Time in Parkinson’s Disease.” Movement Disorders 37 (10): 2163–64. https://doi.org/10.1002/MDS.29166.
[5] Mondal, B., S. Choudhury, K. Chatterjee, R. Banerjee, S. Shubham, M. Baker, and H. Kumar. 2018. “Therapeutic Effect of Non-Invasive Vagus Nerve Stimulation in Gait Disturbance and Freezing in Parkinson’s Disease Patients.” Parkinsonism & Related Disorders 46 (January): e21. https://doi.org/10.1016/j.parkreldis.2017.11.070.
[6] Mondal, Banashree, Supriyo Choudhury, Rebecca Banerjee, Akash Roy, Koustav Chatterjee, Purba Basu, Ravi Singh, et al. 2021. “Non-Invasive Vagus Nerve Stimulation Improves Clinical and Molecular Biomarkers of Parkinson’s Disease in Patients with Freezing of Gait.” NPJ Parkinson’s Disease 7 (1). https://doi.org/10.1038/S41531-021-00190-X.
[7] Cakmak, Yusuf O., Hülya Apaydin, Güneş Kiziltan, Ayşegül Gündüz, Burak Ozsoy, Selim Olcer, Hakan Urey, Ozgur O. Cakmak, Yasemin G. Ozdemir, and Sibel Ertan. 2017. “Rapid Alleviation of Parkinson’s Disease Symptoms via Electrostimulation of Intrinsic Auricular Muscle Zones.” Frontiers in Human Neuroscience 11 (June): 338. https://doi.org/10.3389/fnhum.2017.00338.
To cite this abstract in AMA style:
V. van Midden, Z. Pirtošek, U. Simončič, M. Kojović. The effect of taVNS on gait in advanced Parkinsons disease – a double blind, sham controlled motion sensors study in 30 patients [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/the-effect-of-tavns-on-gait-in-advanced-parkinsons-disease-a-double-blind-sham-controlled-motion-sensors-study-in-30-patients/. Accessed April 27, 2025.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/the-effect-of-tavns-on-gait-in-advanced-parkinsons-disease-a-double-blind-sham-controlled-motion-sensors-study-in-30-patients/