Session Information
Date: Thursday, June 23, 2016
Session Title: Parkinson's disease: Clinical trials, pharmacology and treatment
Session Time: 12:00pm-1:30pm
Location: Exhibit Hall located in Hall B, Level 2
Objective: To assess the tolerability and effect of levodopa-carbidopa intestinal gel (LCIG, designated in the United States as carbidopa-levodopa enteral suspension) on activities of daily living (ADL) in advanced Parkinson’s disease (PD) patients with troublesome motor fluctuations.
Background: LCIG is continuously delivered via percutaneous gastrojejunostomy (PEG-J) in advanced PD patients with motor fluctuations and dyskinesia not adequately reduced by available oral anti-Parkinsonian medications.
Methods: This multinational (7 countries, 35 centres) post-marketing observational study included advanced PD patients characterised by either 2-4 hours “off” time or 2 hours of dyskinesia daily, supported by a Unified Parkinson’s disease Rating Scale (UPDRS) Total Score of ≥40 at baseline. LCIG was titrated via a nasojejunal (NJ) tube, then administered via PEG-J for 12 months. ADL and quality of life were assessed by the UPDRS Part II total score and 8-item Parkinson’s disease Questionnaire (PDQ-8) summary index, respectively. Adverse events (AEs) were collected.
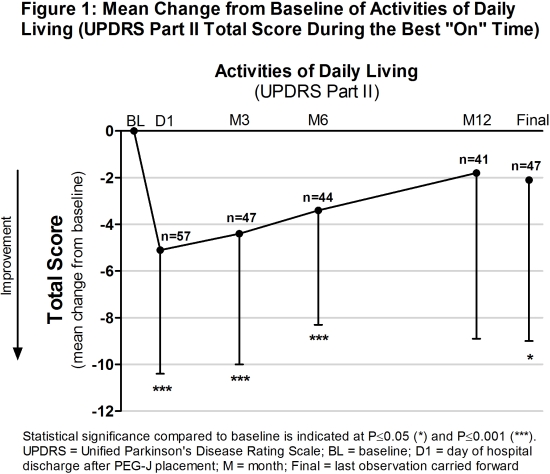
Results: Sixty-five patients were enrolled, 58 out of 64 patients (91%) who were treated with LCIG via NJ continued treatment via PEG-J (1 discontinued before titration), and 41 completed the study. At baseline, patients had PD for a mean of 13.9 years (Table 1). Patients had improvements in activities of daily living in the first 6 months and at final (Figure 1), and in quality of life (PDQ-8 summary index) at every time point. One-third of patients had an AE (Table 2), of which, 8 (12.5%) had an AE with a reasonable possibility of being related to LCIG, as rated by the study investigator.
| Demographic or Characteristic | n (% of N=64) | Mean (SD) | |
| Gender | Male | 39 (61) | |
| Female | 25 (39) | ||
| Age, years | 70.4 (7.8) | ||
| Race | Caucasian | 64 (100) | |
| Parkinson’s disease duration, years | 13.9 (5.4) | ||
| Previous PD therapy | Oral levodopa | 64 (100) | |
| Dopamine agonist | 60 (94) | ||
| COMT inhibitor | 45 (70) | ||
| MAO-B inhibitor | 38 (59) | ||
| Amantadine | 31 (48) | ||
| Rotigotine | 25 (39) | ||
| Apomorphine s.c. | 12 (19) | ||
| UPDRS Part II, total score during “on” time | 18.1 (8.0) | ||
| Patients with: | n (% of N=64) |
| Any adverse event (AE) | 21 (33) |
| Any serious AE | 14 (22) |
| Any AE leading to discontinuation of LCIG | 7 (11) |
| Death | 2 (3.1)a |
| AEs occurring in ≥2 patients by preferred term: | |
| Stoma site infection [ensp] [ensp] | 3 (4.7) |
| Therapy cessationb | 3 (4.7) |
| Psychotic disorder | 2 (3.1) |
a. Both patients had AEs that had no reasonable possibility of being related to the treatment, as rated by the investigator.
b. This preferred term was under the surgical and medical procedures system organ class.
Conclusions: LCIG led to significant improvements in activities of daily living in the first 6 months of infusion in advanced PD patients. The safety results were consistent with the established safety profile of LCIG. (ref 1). References: 1. Fernandez et al. Mov Disord. 2015; 30(4):500-9.
To cite this abstract in AMA style:
R. Krüger, P. Lingor, T. Doskas, J. Henselmans, E.H. Danielsen, O. de Fabregues, A. Stefani, S.C. Sensken, J.C. Parra Riaza, K. Onuk, A. Yegin. The effect of levodopa-carbidopa intestinal gel on activities of daily living: Results from the observational MONOTREAT study in advanced Parkinson’s disease patients [abstract]. Mov Disord. 2016; 31 (suppl 2). https://www.mdsabstracts.org/abstract/the-effect-of-levodopa-carbidopa-intestinal-gel-on-activities-of-daily-living-results-from-the-observational-monotreat-study-in-advanced-parkinsons-disease-patients/. Accessed December 26, 2025.« Back to 2016 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/the-effect-of-levodopa-carbidopa-intestinal-gel-on-activities-of-daily-living-results-from-the-observational-monotreat-study-in-advanced-parkinsons-disease-patients/
