Session Information
Date: Tuesday, September 24, 2019
Session Title: Spasticity
Session Time: 1:45pm-3:15pm
Location: Les Muses Terrace, Level 3
Objective: To report primary outcomes from ENGAGE, an international phase 3b/4, prospective, single-arm, open-label study (NCT02969356).
Background: ENGAGE assessed effects of Guided Self-rehabilitation Contracts (GSC) alongside co-injection of abobotulinumtoxinA (aboBoNT-A) into upper (UL) and lower limbs (LL) on active movements in adult patients with chronic hemiparesis resulting from acquired brain injury. GSC are a diary-based and antagonist-targeting strategy, in which therapists identify target muscles against which to prescribe patients daily home-based self-stretching and active training programmes. Voluntary movements were measured using a goniometer to determine composite active range of motion (CXA), a tool that correlates with functional outcomes.
Method: Patients with chronic spastic hemiparesis, stratified with UL or LL as primary treatment target (PTT), received personalised GSC together with 2 consecutive injections of aboBoNT-A 1500 U across PTT and non-PTT limbs. Primary efficacy endpoint was proportion of responders in the PTT (improvement in CXA of ≥35° or 5° in UL or LL, respectively) at Cycle 2 Week 6.
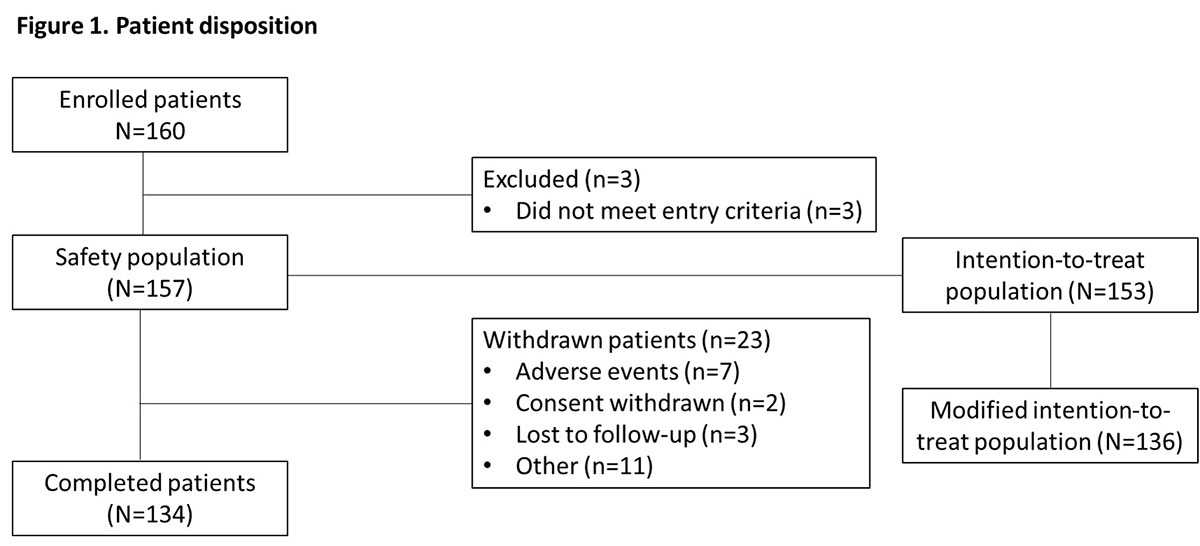
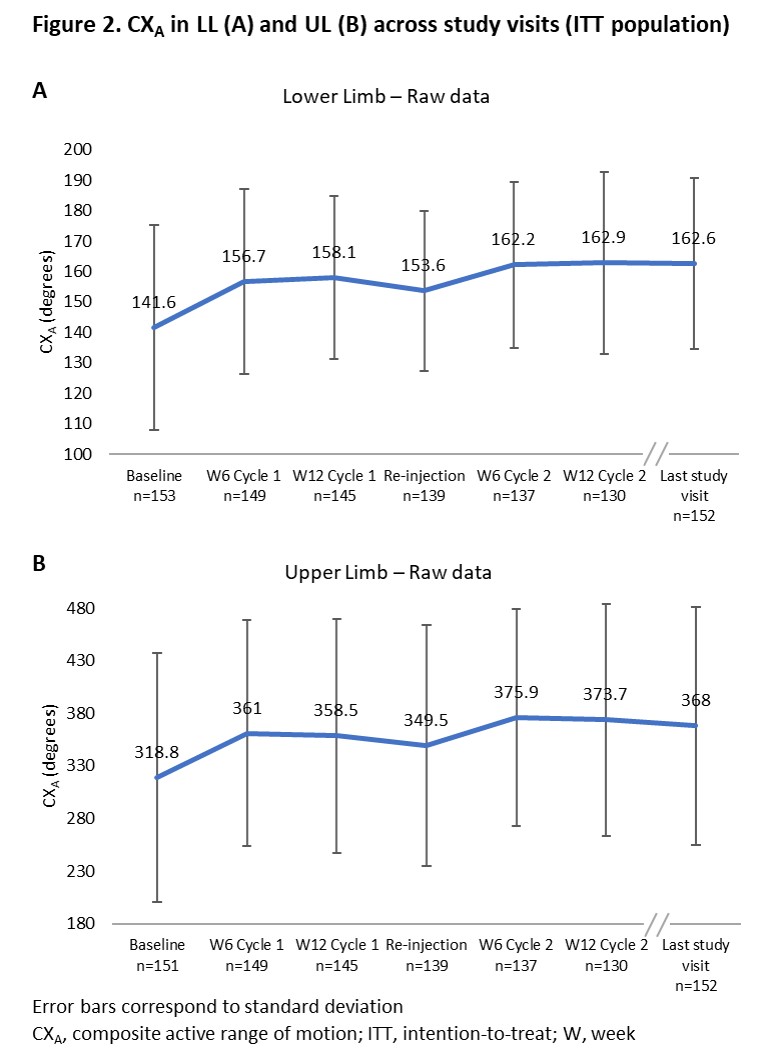
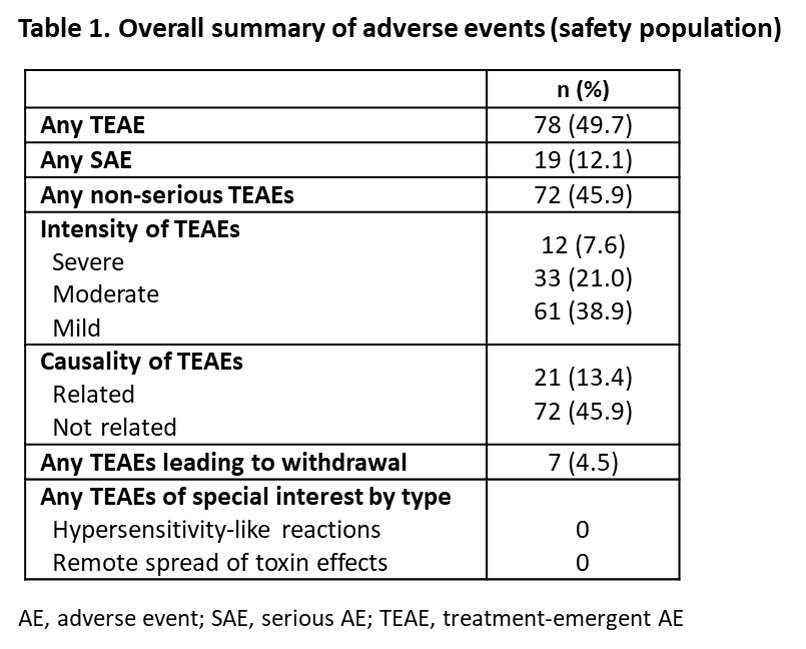
Results: Of 160 patients enrolled, 153 received treatment and were included in the intention-to-treat (ITT) population (mean [SD] age, 52.9 [12.6] years; aetiology, 90.8% stroke) and 134 completed the study (Figure 1). The proportion of patients split by PTT was 52.3% versus 47.7% for UL and LL, respectively. In the modified ITT population (n=136), mean (SD) overall compliance to GSC was 92.8% (9.9) and 98 patients (72.1% [95% CI 64.0, 78.9]) achieved the primary efficacy endpoint. A greater responder rate was observed in patients with LL as PTT (83.1% [95% CI 72.0, 90.5]) versus those with UL as PTT (62.0% [95% CI 50.3, 72.4]). Mean (SD) CXA increased from 141.6° (33.7) at baseline to 162.6° (28.1) at last study visit in LL, and from 318.8° (118.3) to 368.0° (112.8), respectively, in UL (Figure 2). Safety data were in line with the known profile of aboBoNT-A; 78 (49.7%) patients reported treatment-emergent adverse events (Table 1).
Conclusion: In this single-arm, open-label study in patients with chronic spastic hemiparesis, the combination of GSC with aboBoNT-A simultaneously injected into UL and LL is associated with improvement of CXA. [figure1] [figure2] [table 1]
To cite this abstract in AMA style:
J-M. Gracies, G. Francisco, R. Jech, S. Khatkova, C. Rios, P. Maisonobe. The effect of Guided Self-rehabilitation Contracts combined with simultaneous injections of abobotulinumtoxinA into upper and lower limbs on voluntary movements in adults with spastic hemiparesis [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/the-effect-of-guided-self-rehabilitation-contracts-combined-with-simultaneous-injections-of-abobotulinumtoxina-into-upper-and-lower-limbs-on-voluntary-movements-in-adults-with-spastic-hemiparesis/. Accessed December 18, 2025.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/the-effect-of-guided-self-rehabilitation-contracts-combined-with-simultaneous-injections-of-abobotulinumtoxina-into-upper-and-lower-limbs-on-voluntary-movements-in-adults-with-spastic-hemiparesis/