Category: Neurophysiology (Non-PD)
Objective: To describe a novel motor cortex (M1) electroencephalogram (EEG) abnormality distinct to celiac disease (CD)-related cortical myoclonus and to explore its relationship with cerebellar-M1 connectivity.
Background: CD is associated with cortical hyperexcitability [1] and a number of neurological manifestations including cortical myoclonus, with or without ataxia [2]. The mechanism underlying cortical hyperexcitability in CD is unknown. A variety of non-specific EEG abnormalities have been described but no distinct CD-related EEG pattern has been reported [3].
Method: We describe the clinical and neurophysiological characteristics of 3 cases of CD-associated cortical myoclonus including EEG, magnetoencephalography (MEG), and transcranial magnetic stimulation (TMS).
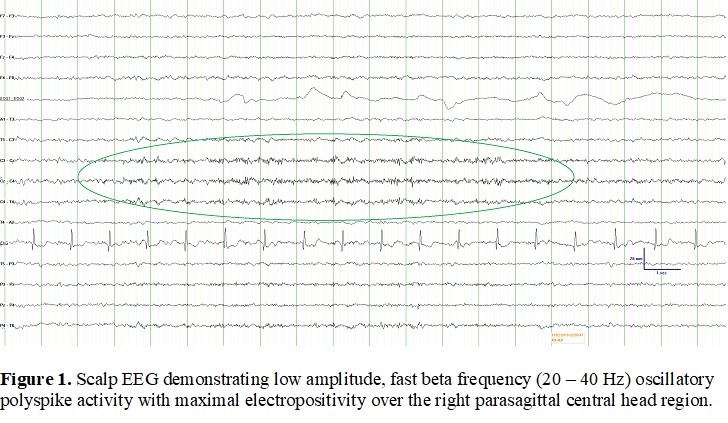
Results: EEG in all cases demonstrated low amplitude (20-40 µV), electropositive fast beta frequency (20-40 Hz) oscillatory polyspike and polyspike and slow wave activity over the central head region, corresponding to M1 contralateral to the myoclonic limb [Figure 1]. Jerk-locked back-averaging demonstrated a preceding EEG-MEG cortical potential; MEG source localization (dipole mapping and beamformer analysis) revealed an identical cortical generator situated in the posterior wall of the precentral gyrus for both the back-averaged potential and the electropositive phase of the spontaneous oscillatory abnormality. In one patient, cerebellar inhibition of M1 was physiologically normal and did not change after cerebellar intermittent theta burst stimulation. Transection of the localized cortex in one patient resulted in cessation of myoclonus.
Conclusion: The finding of oscillatory, low amplitude, electropositive high frequency EEG polyspike activity over the central head region corresponding to M1 may be pathognomonic of CD-related cortical myoclonus and its presence may serve as an important diagnostic clue for this condition. These oscillatory rhythms are consistent with prior descriptions of CD-related cortical hyperexcitability, which may not necessarily result from direct cerebellar disinhibition of the contralateral M1.
References: [1] Pennisi G, Lanza G, Giuffrida S, Vinciguerra L, Puglisi V, Cantone M, et al. Excitability of the motor cortex in de novo patients with celiac disease. PLoS ONE 2014;9:e102790. [2] Sarrigiannis PG, Hoggard N, Aeschlimann D, Sanders DS, Grunewald RA, Unwin ZC, et al. Myoclonus ataxia and refractory coeliac disease. Cerebellum Ataxias 2014;1:11. [3] Parisi P, Principessa L, Ferretti A, D’Onofrio D, Del Giudice E, Pacchiarotti C, et al. “EEG abnormalities” may represent a confounding factor in celiac disease: a 4-year follow-up family report. Epilepsy Behav. Case Rep. 2014;2:40–42. Additional References Ashby P, Chen R, Wennberg R, Lozano AM, Lang AE. Cortical reflex myoclonus studied with cortical electrodes. Clin Neurophysiol 1999 Sep;110(9):1521-30. Bella R, Lanza G, Cantone M, Giuffrida S, Puglisi V, Vinciguerra L, et al. Effect of a gluten-free diet on cortical excitability in adults with Celiac disease. PLoS ONE 2015;10:e0129218. Bhatia KP, Brown P, Gregory R, Lennox GG, Manji H, Thompson PD, et al. Progressive myoclonic ataxia associated with coeliac disease. The myoclonus is of cortical origin, but the pathology is in the cerebellum. Brain 1995 Oct;118 ( Pt 5):1087-93. Burk K, Farecki ML, Lamprecht G, et al. Neurological symptoms in patients with biopsy proven celiac disease. Mov Disord 2009;24(16): 2358–2362. Engel J, Jr., Rapin I, Giblin DR. Electrophysiological studies in two patients with cherry red spot–myoclonus syndrome. Epilepsia 1977 Mar;18(1):73-87. Groth JD, Sahin M. High frequency synchrony in the cerebellar cortex during goal directed movement. Front Syst Neurosci 2015;9:98. Hadjivassiliou M, Aeschlimann P, Strigun A, Sanders DS, Woodroofe N, Aeschlimann D. Autoantibodies in gluten ataxia recognize a novel neuronal transglutaminase. Ann Neurol 2008;64(3):332–343. Jesus S, Latorre A, Vinuela A, Fahn S, Bhatia K, Balint B. Stimulus sensitive foot myoclonus: a clue to celiac disease. Mov Disord Clin Pract 2019;6(4):320-323. Labate A, Gambardella A, Messina D, Tammaro S, Le Piane E, Pirritano D, et al. Silent celiac disease in patients with childhood localization-related epilepsies. Epilepsia 2001;42:1153–55. Lu CS, Thompson PD, Quinn NP, Parkes JD, Marsden CD. Ramsay Hunt syndrome and coeliac disease: a new association? Mov Disord 1986;1(3):209-19. Matsuo F, Knott JR. Focal positive spikes in electroencephalography. Electroencephalogr Clin Neurophysiol 1977 Jan;42(1):15-25. Middleton SJ, Racca C, Cunningham MO, Traub RD, Monyer H, Knopfel T, et al. High-frequency network oscillations in cerebellar cortex. Neuron 2008;58(5):763-74. Paramanandam V, Hadjivassiliou M, Olszewska DA, Munteanu T, Williams L, Magennis B, et al. Reversible Corticobasal Syndrome due to Coeliac Disease. Mov Disord Clin Pract 2018 Sep;5(5):551-4. Thompson PD, Day BL, Rothwell JC, Brown P, Britton TC, Marsden CD. The myoclonus in corticobasal degeneration. Evidence for two forms of cortical reflex myoclonus. Brain 1994 Oct;117 ( Pt 5):1197-207. Tijssen MA, Thom M, Ellison DW, Wilkins P, Barnes D, Thompson PD, et al. Cortical myoclonus and cerebellar pathology. Neurology 2000;54:1350–56. Vinagre-Aragon A, Zis P, Grunewald RA, Hadjivassilou M. Movement disorders related to gluten sensitivity: A systematic review. Nutrients 2019;10(8):1034-47.
To cite this abstract in AMA style:
E. Swinkin, K. Lizarraga, M. Algarni, L. Dominguez, J. Baarbe, J. Saravanamuttu, R. Chen, E. Slow, A. Lang, R. Wennberg. The distinct EEG signature of celiac disease-related cortical myoclonus [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/the-distinct-eeg-signature-of-celiac-disease-related-cortical-myoclonus/. Accessed December 31, 2025.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/the-distinct-eeg-signature-of-celiac-disease-related-cortical-myoclonus/