Session Information
Date: Tuesday, June 21, 2016
Session Title: Restless legs syndrome and other sleep disorders
Session Time: 12:30pm-2:00pm
Location: Exhibit Hall located in Hall B, Level 2
Objective: To quantify the magnitude of the placebo response in restless legs syndrome (RLS).
Background: RLS is a common sensorimotor disorder characterized by an urge to move the legs. Previous research in the field suggests that a substantial placebo response is present, leading to relevant symptom relief independent of an active intervention.
Methods: Search methods: The bibliographic databases MEDLINE, EMBASE and CENTRAL, as well as clinical trials registries, were searched in October 2015. Reference lists of retrieved articles were cross-checked for additional eligible studies. Selection criteria: Randomized, double-blind, placebo-controlled trials studying RLS were included if at least one of the outcomes of interest, as measured by a validated instrument, was extractable in the placebo arm. Data collection and analysis: Two reviewers independently extracted data and assessed risk of bias. Authors were contacted for missing data. two separate meta-analyses were performed for each continuous outcome using two effect measures: mean change from baseline, presented as natural units of the most familiar scale, and effect size. Protocol registration number: CRD42015027992.
Results: Eighty-five trials were included (5037 patients on placebo). 64 studies reported a validated RLS severity assessment (4111 patients), 62 of which applied the International RLS Study Group rating scale (IRLS). The mean change from baseline in IRLS was -6.6 (95% CI: -8.3 to -4.9).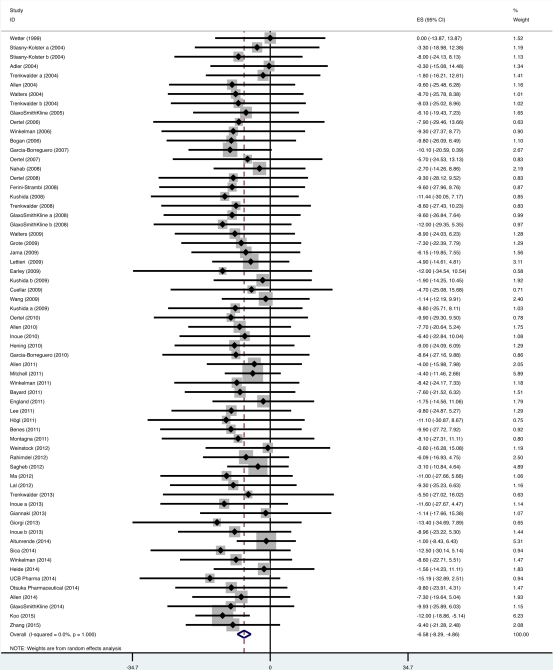 Disease severity did not influence the placebo response. There was an increased magnitude of the placebo response according to study duration. No significant difference in placebo response (using mean change from baseline) was found in: parallel vs. crossover design; pharmacological vs. non-pharmacological interventions; oral vs. non-oral administration; idiopathic vs. secondary RLS; and low vs. unclear risk of bias. The nocebo response, adverse events in placebo arm, was 45.4%. For the periodic limb movement index we found a response very close to zero, unlike the other outcomes studied.
Disease severity did not influence the placebo response. There was an increased magnitude of the placebo response according to study duration. No significant difference in placebo response (using mean change from baseline) was found in: parallel vs. crossover design; pharmacological vs. non-pharmacological interventions; oral vs. non-oral administration; idiopathic vs. secondary RLS; and low vs. unclear risk of bias. The nocebo response, adverse events in placebo arm, was 45.4%. For the periodic limb movement index we found a response very close to zero, unlike the other outcomes studied.
Conclusions: The magnitude of the placebo response in RLS is substantial, being above the threshold for clinical significance, and with very low heterogeneity. Besides study duration, we found no other determinants of the placebo response. It was also documented a higher than expected frequency of adverse events in the placebo arm.
To cite this abstract in AMA style:
F.B. Rodrigues, M. Silva, G.S. Duarte, R. Camara, R. Fernandes, D. Abreu, J. Costa, J.J. Ferreira. Systematic review and meta-analysis of randomized controlled trials to evaluate placebo response in restless legs syndrome [abstract]. Mov Disord. 2016; 31 (suppl 2). https://www.mdsabstracts.org/abstract/systematic-review-and-meta-analysis-of-randomized-controlled-trials-to-evaluate-placebo-response-in-restless-legs-syndrome/. Accessed April 20, 2025.« Back to 2016 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/systematic-review-and-meta-analysis-of-randomized-controlled-trials-to-evaluate-placebo-response-in-restless-legs-syndrome/
