Session Information
Date: Saturday, October 6, 2018
Session Title: Parkinson’s Disease: Clinical Trials, Pharmacology And Treatment
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
Objective: To describe the cohort screened and randomized into the Study of Urate Elevation in Parkinson’s Disease, phase 3 (SURE-PD3) trial (NCT02642393).
Background: The SURE-PD3 trial is a randomized, double-blind, placebo-controlled, phase 3 trial of urate-elevating oral inosine to slow clinical decline in early PD. A disease-modifying effect of urate in PD is suggested by evidence from laboratory (neuroprotection), epidemiological (inverse risk factor), and clinical biomarker studies. Elevated urate predicted slower PD progression in prior clinical trial cohorts, including DATATOP[1] and PRECEPT[2]. SURE-PD3 was designed to match key features of these earlier trials to test whether the association between elevated urate and slower progression is causal.
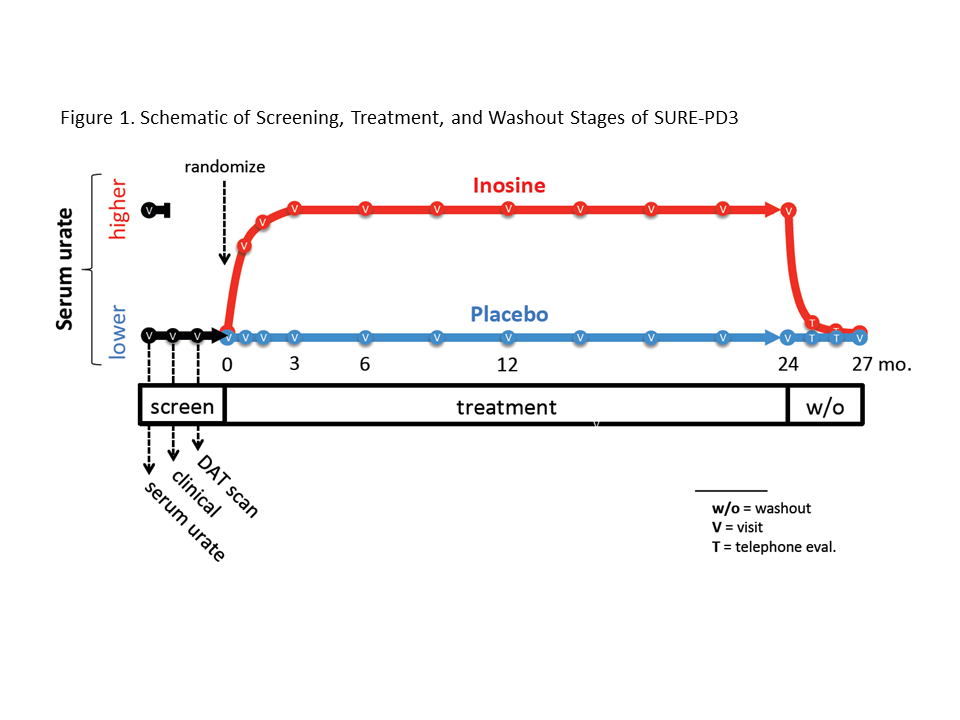
Methods: Key eligibility criteria were diagnosis of idiopathic PD within 3 years, not requiring or taking antiparkinsonian medication other than a stable dose of an MAO-B inhibitor, serum urate ≤5.7 mg/dL, evidence of dopaminergic deficit by DAT scan, and absence of risk factors for gout or urolithiasis. The design included a 3-stage screening process, 1:1 randomization to placebo or oral inosine titrated to elevate serum urate to 7.1 to 8.0 mg/dL over 24 months, and a 3-month washout to test durability of any benefit (Figure 1).
Results: Between Jul 2016 and Dec 2017, 587 individuals were consented and screened at 60 PSG sites, with 186 (32%) excluded after initial screening due to serum urate >5.7 mg/dL. Of 336 screened by DAT scan, 30 (9%) were excluded due to lack of evidence of dopaminergic deficit. After evaluating all eligibility criteria, 298 participants were randomized, mean (SD) age was 63.3 (9.6) years at baseline, 49% female, mod H&Y 1.7 (0.5), mod S&E ADL 93.8 (7.2), 38% on an MAO-B inhibitor, BMI 26.6 (4.9) kg/m2, 32%/3% former/current smokers, MMSE 29.3 (1.0), MDS-UPDRS I-III 32.9 (12.5), and serum urate 4.2 (0.8) mg/dL among women and 4.9 (0.6) mg/dL among men. By design, these characteristics match DATATOP and PRECEPT except for occurrence of MAO-B inhibitor use, lower mean serum urate concentrations and a correspondingly higher proportion of women (~35% in the earlier trials).
Conclusions: The cohort screened was typical of de novo PD trial patients. Equal enrollment of men and women resulted from counter-balancing effects of lower serum urate and lower PD prevalence among women. Results from the trial are expected in early 2020.
References: [1] Ascherio A, LeWitt PA, Xu K, Eberly S, Watts A, Matson WR, Marras C, Kieburtz K, Rudolph, A, Bogdanov M, Schwid SR, Tennis M, Tanner CM, Beal MF, Lang AE, Oakes D, Fahn S, Shoulson I, Schwarzschild MA on behalf of the PSG DATATOP investigators. (2009) Urate predicts rate of clinical decline in Parkinson disease. Arch. Neurol. 66:1460-1468. [2] Schwarzschild MA, Schwid SR, Marek K, Watts A, Lang AE, Oakes D, Shoulson I, & Ascherio A, and the Parkinson Study Group PRECEPT Investigators. Serum urate as a predictor of clinical and radiographic progression in Parkinson’s disease. (2008) Arch Neurol. 65:716-723.
To cite this abstract in AMA style:
E. Macklin, A. Videnovic, C. Lungu, C. Kamp, C. Casaceli, D. Oakes, A. Ascherio, M. Schwarzschild; on behalf of the Parkinson Study Group SURE-PD3_Investigators. SURE-PD3 Trial Participant Features prior to Randomization [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/sure-pd3-trial-participant-features-prior-to-randomization/. Accessed December 15, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/sure-pd3-trial-participant-features-prior-to-randomization/