Category: Parkinson's Disease: Non-Motor Symptoms
Objective: To determine whether orthostatic hypotension (OH) and/or supine hypertension (SH) are longitudinally associated with cognitive decline in Parkinson disease (PD).
Background: Cognitive impairment is associated with OH and SH in PD. Whether this represents a particular PD phenotype with more diffuse Lewy body pathology, affecting both cognition and autonomic function, or whether OH and SH impact cognition via hypoxia-associated atrophy or white matter disease remains unclear [1-5]. If the latter, treatment of OH and/or SH should reduce cognitive decline. First, however, it is critical to clarify the relative contributions of OH and SH to cognitive decline, as either may be a confounder and pharmacologic management of one may worsen the other [6].
Method: We evaluated data from participants with de novo PD enrolled in Parkinson Progression Markers Initiative with a baseline visit containing all relevant variables [7]. OH and SH were defined from vital sign measurement while cognitive function across different domains was defined from a standardized battery [8-10]. Longitudinal associations between annual presence/absence of OH/SH and change in cognitive scores up to 9 years were determined using linear mixed effects models, adjusting for appropriate baseline and visit-updated variables (p≤0.008 was considered significant after correction for multiple comparisons).
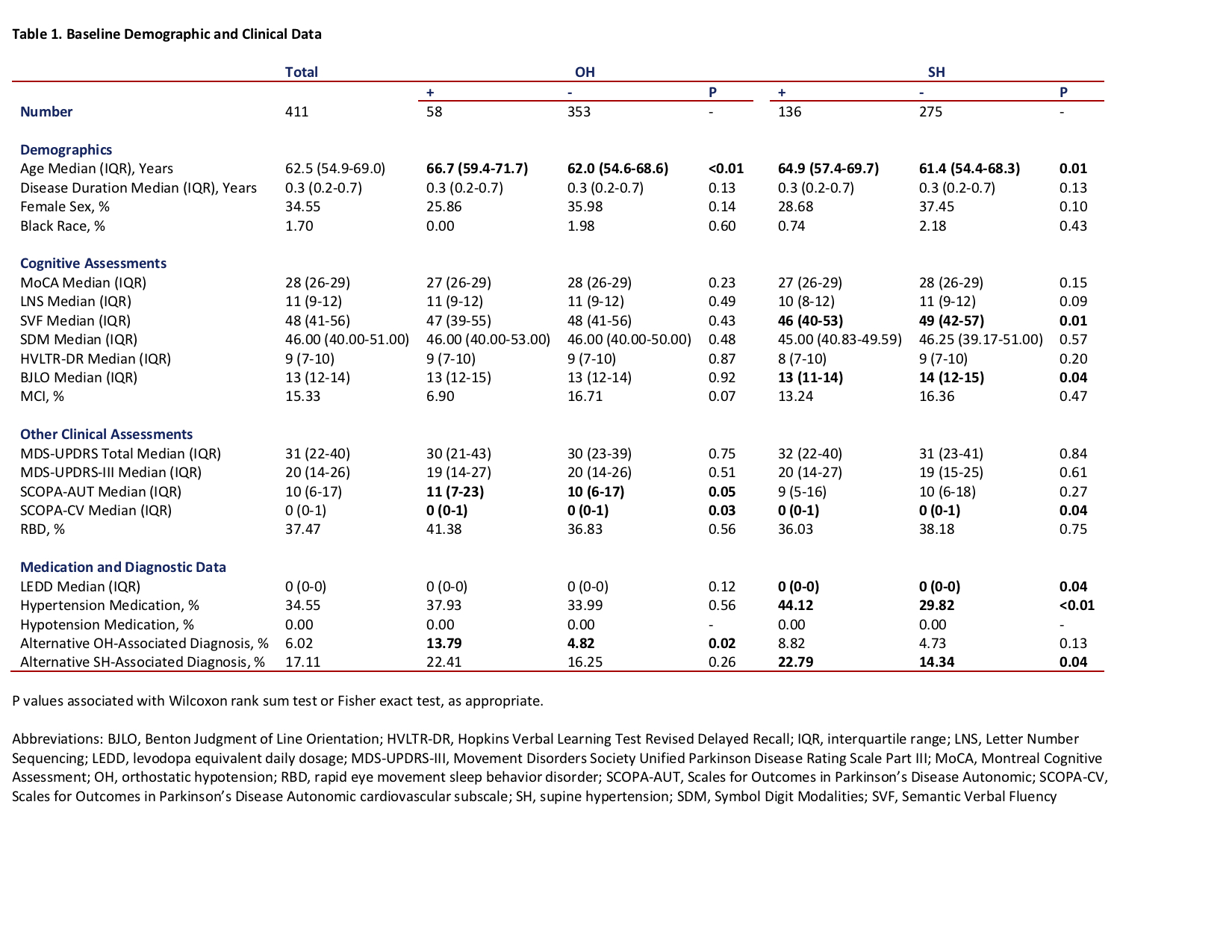
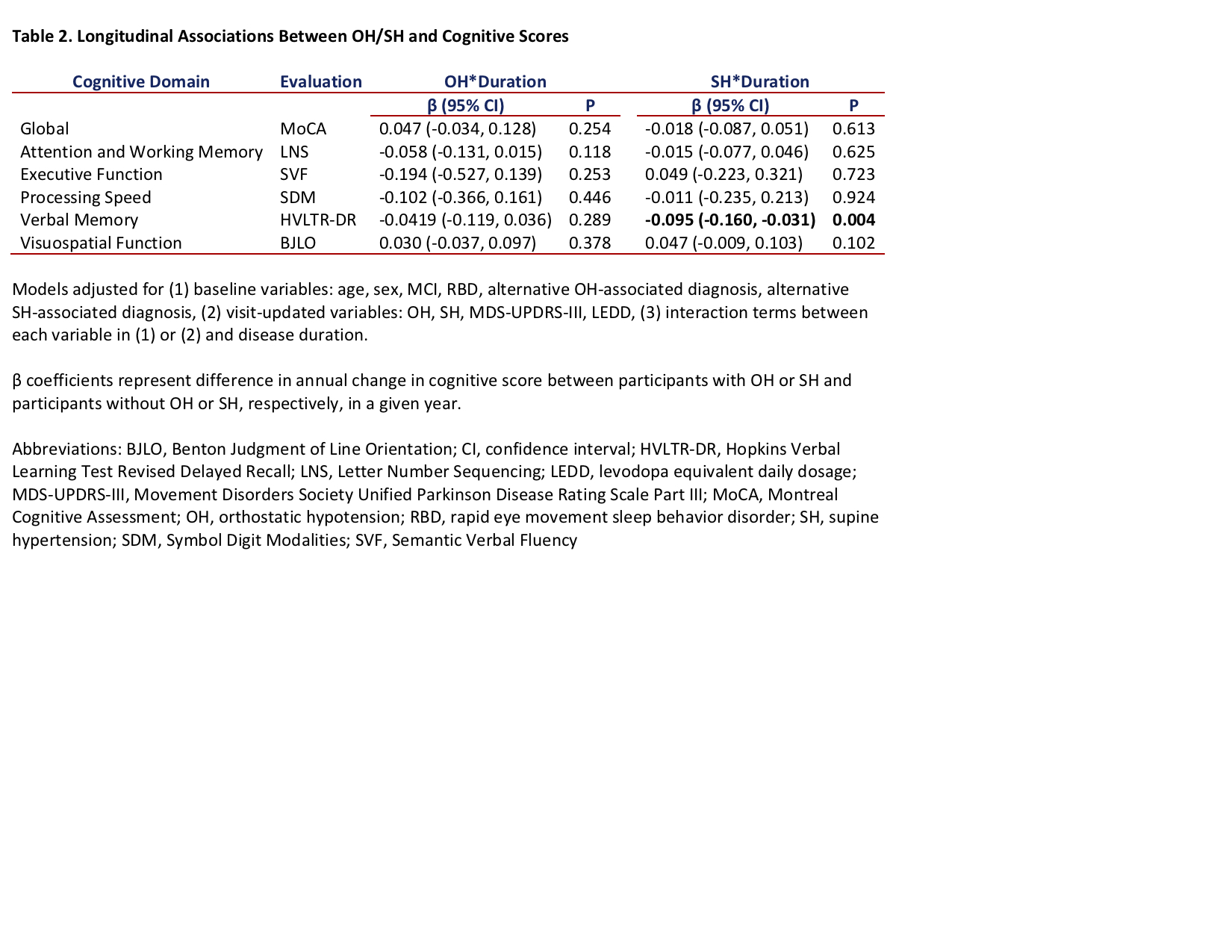
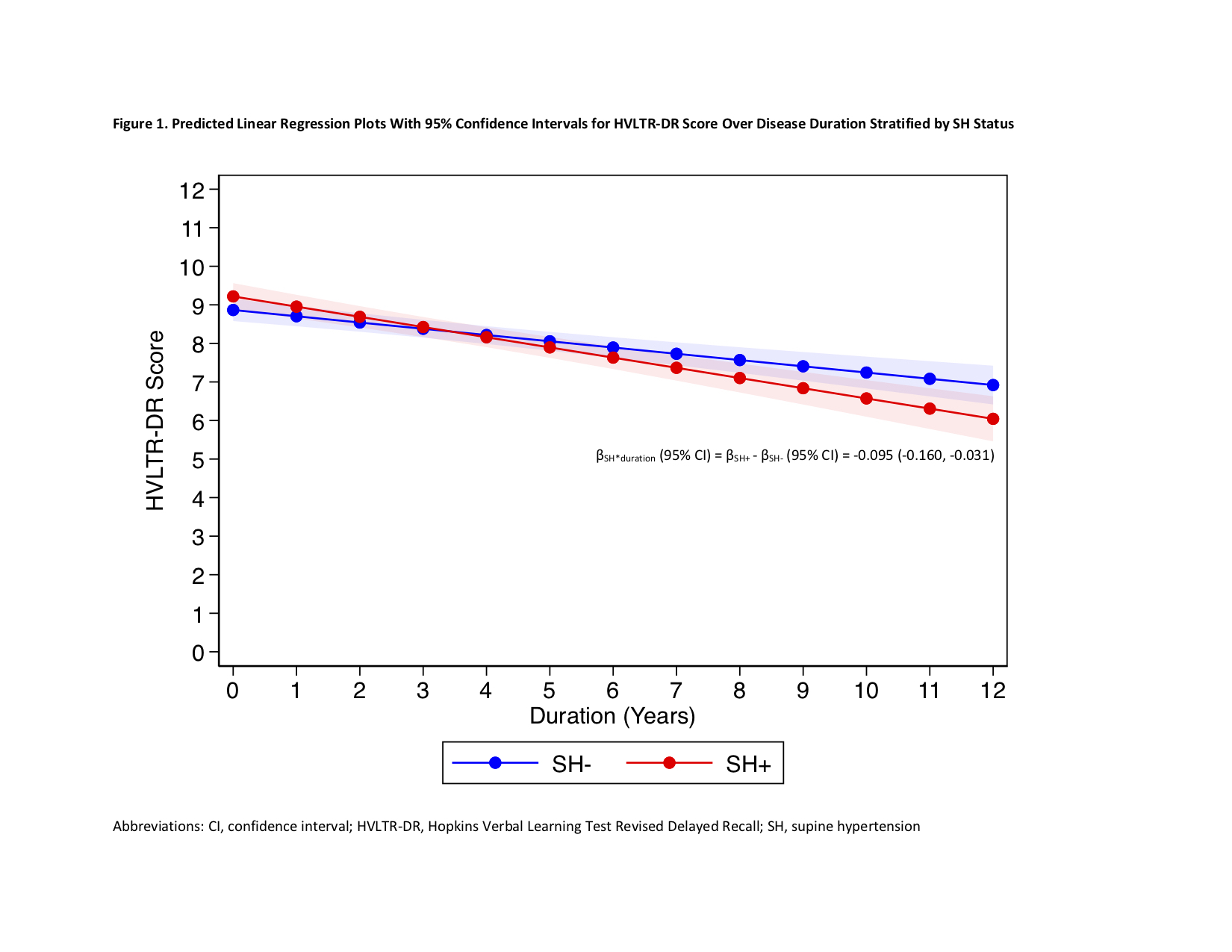
Results: A total of 411 participants were included, with median baseline age 62.5 years and 34.55% female. [table1] Independent of OH and other variables, annual presence of SH was associated with greater annual decline in Hopkins Verbal Learning Test Revised Delayed Recall; the mean β coefficient (95% confidence interval) for SH*duration, which indicates difference in annual score change between participants with versus without SH, was -0.095 (-0.160, -0.031), p=0.004. Annual presence of OH was not associated with change in any cognitive scores. [table2] [figure1]
Conclusion: Longitudinal presence of SH, but not OH, was associated with greater decline in cognition, particularly verbal memory. Prospective studies incorporating neuroimaging are needed to further evaluate whether this longitudinal relationship is due to more diffuse Lewy body disease or white matter disease. If the latter, then identification and treatment of SH should reduce cognitive decline.
References: 1. Fereshtehnejad SM, Romenets SR, Anang JB, Latreille V, Gagnon JF, Postuma RB: New Clinical Subtypes of Parkinson Disease and Their Longitudinal Progression: A Prospective Cohort Comparison With Other Phenotypes. JAMA Neurol 2015, 72(8):863-873.
2. Kim JS, Oh YS, Lee KS, Kim YI, Yang DW, Goldstein DS: Association of cognitive dysfunction with neurocirculatory abnormalities in early Parkinson disease. Neurology 2012, 79(13):1323-1331.
3. Dadar M, Fereshtehnejad SM, Zeighami Y, Dagher A, Postuma RB, Collins DL: White Matter Hyperintensities Mediate Impact of Dysautonomia on Cognition in Parkinson’s Disease. Mov Disord Clin Pract 2020, 7(6):639-647.
4. Pilotto A, Romagnolo A, Scalvini A, Masellis M, Shimo Y, Bonanni L, Camicioli R, Wang LL, Dwivedi AK, Longardner K et al: Association of Orthostatic Hypotension With Cerebral Atrophy in Patients With Lewy Body Disorders. Neurology 2021, 97(8):e814-e824.
5. Ruiz Barrio I, Miki Y, Jaunmuktane ZT, Warner T, De Pablo-Fernandez E: Association Between Orthostatic Hypotension and Dementia in Patients With Parkinson Disease and Multiple System Atrophy. Neurology 2023, 100(10):e998-e1008.
6. Espay AJ, LeWitt PA, Hauser RA, Merola A, Masellis M, Lang AE: Neurogenic orthostatic hypotension and supine hypertension in Parkinson’s disease and related synucleinopathies: prioritisation of treatment targets. Lancet Neurol 2016, 15(9):954-966.
7. Parkinson Progression Marker I: The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol 2011, 95(4):629-635.
8. Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH et al: Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 2011, 21(2):69-72.
9. Fanciulli A, Jordan J, Biaggioni I, Calandra-Buonaura G, Cheshire WP, Cortelli P, Eschlboeck S, Grassi G, Hilz MJ, Kaufmann H et al: Consensus statement on the definition of neurogenic supine hypertension in cardiovascular autonomic failure by the American Autonomic Society (AAS) and the European Federation of Autonomic Societies (EFAS) : Endorsed by the European Academy of Neurology (EAN) and the European Society of Hypertension (ESH). Clin Auton Res 2018, 28(4):355-362.
10. Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, Mollenhauer B, Adler CH, Marder K, Williams-Gray CH et al: Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord 2012, 27(3):349-356.
To cite this abstract in AMA style:
C. Miller-Patterson, J. Hsu, M. Barrett, L. Cloud, T. Chelimsky. Supine hypertension is longitudinally associated with cognitive decline in Parkinson disease independent of orthostatic hypotension [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/supine-hypertension-is-longitudinally-associated-with-cognitive-decline-in-parkinson-disease-independent-of-orthostatic-hypotension/. Accessed December 13, 2025.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/supine-hypertension-is-longitudinally-associated-with-cognitive-decline-in-parkinson-disease-independent-of-orthostatic-hypotension/