Category: Parkinson's Disease: Neuroimaging
Objective: To investigate the voxel-wise spatiotemporal changes in neuromelanin-sensitive MRI (NM-MRI) signal in the substantia nigra and their relation to clinical scores of disease severity in patients with early or more advanced idiopathic Parkinson’s disease (PD) and patients with idiopathic rapid eye movement sleep behavior disorder (iRBD) compared to healthy controls (HCs).
Background: NM-MRI produces images where the substantia nigra (SN) appears hyperintense, because the neuromelanin-iron complex is paramagnetic [1]. NM-MRI can be used to analyze changes in SN shape or signal-to-noise ratio (SNR).
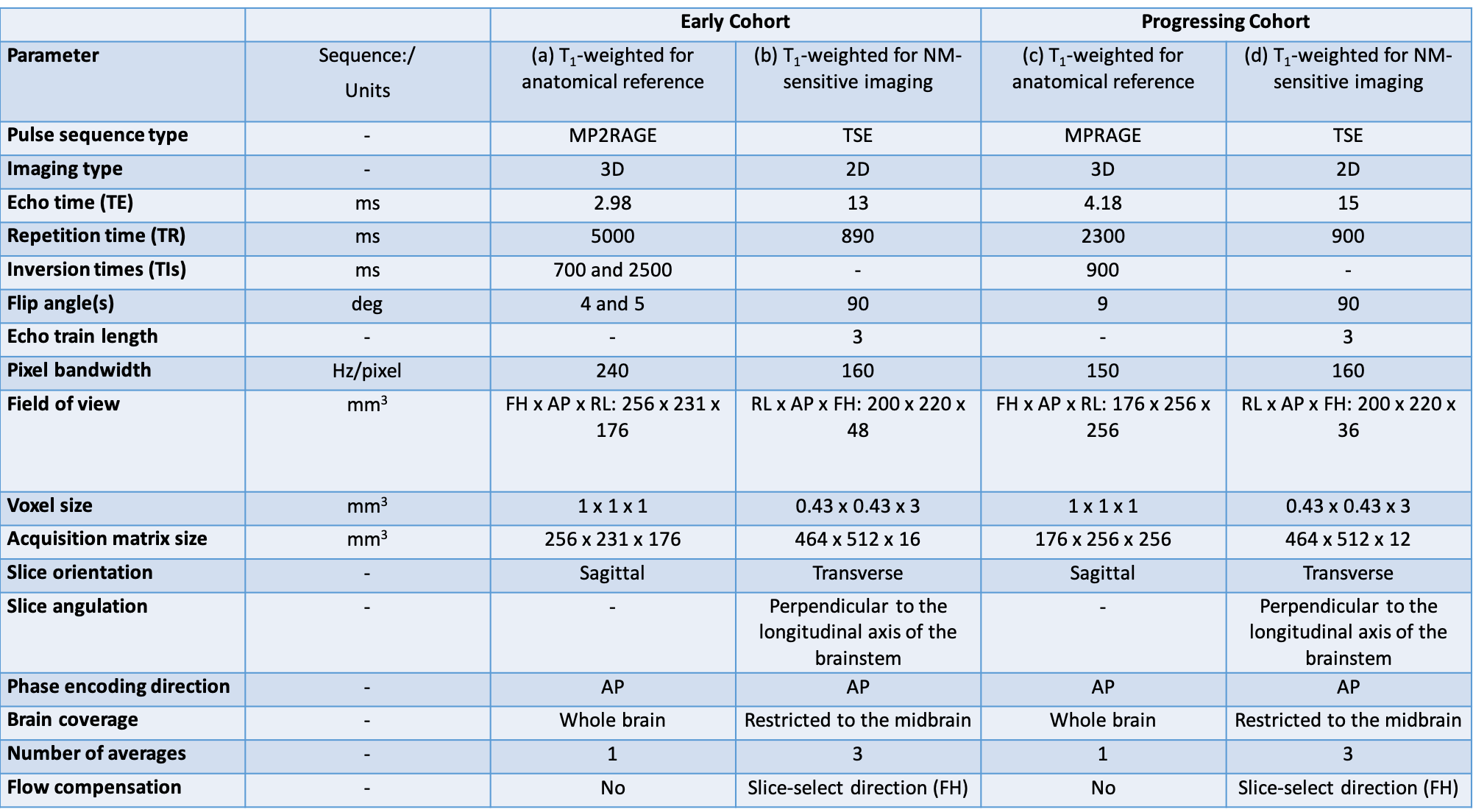
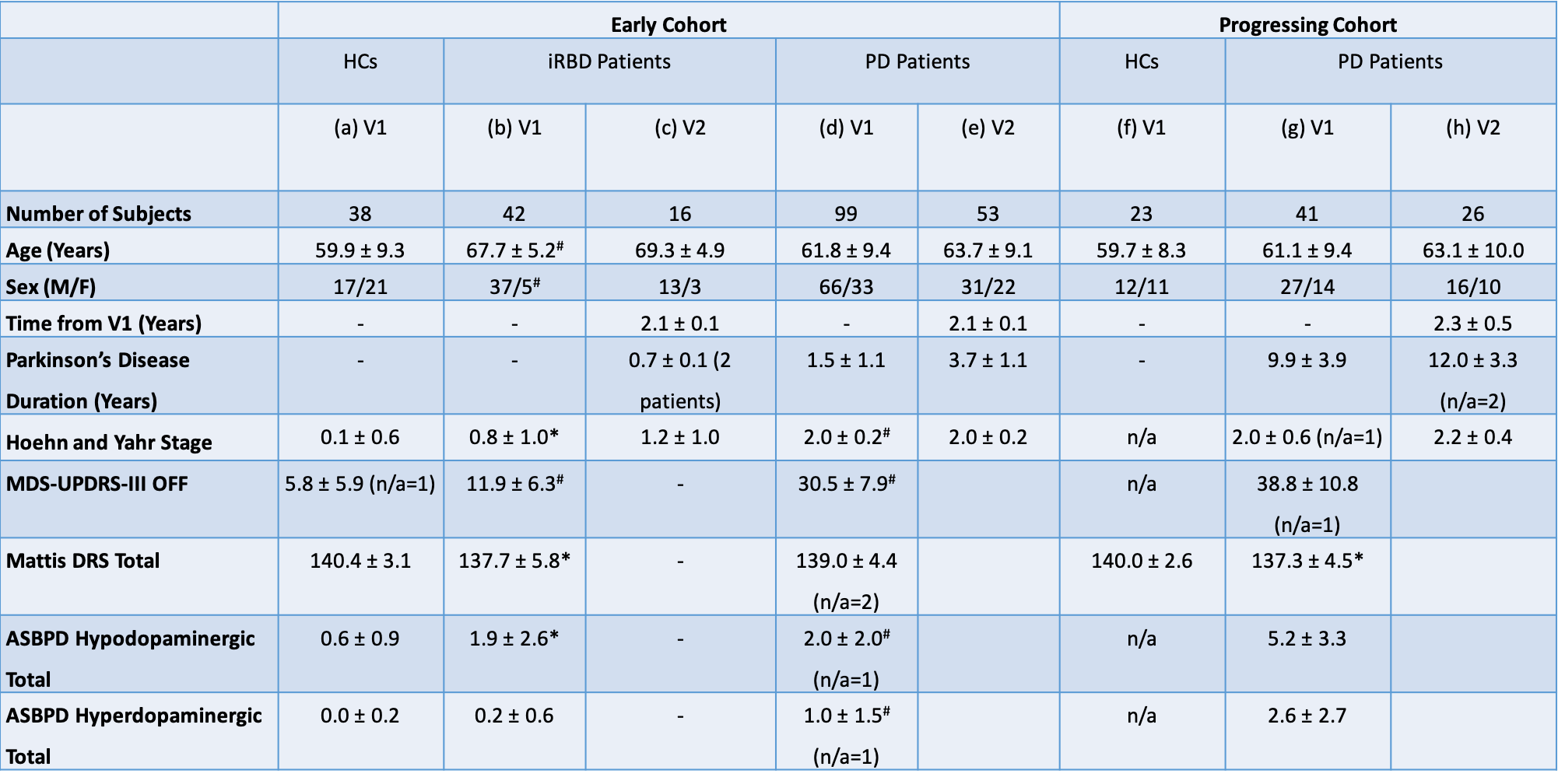
Method: We performed a voxel-wise analysis of longitudinal NM-MRI data [table1] from two cohorts (early and progressing) including HCs, patients with iRBD and PD [table2] by aligning each subject to a study-specific group-balanced brain template. First, we evaluated the local spatiotemporal variations in SN volume by manually segmenting the SN on each subject’s NM-MRI and calculating maps of probability of SN segmentation for HCs, iRBD and PD patients at progressing stages of disease. Second, for each voxel in the binarized SN probability map of HCs, we evaluated the correlation between the SNR of NM-MRI with motor (MDS-UPDRS-III in off, [2]), cognitive (Mattis Dementia Rating Scale, [3, 4]) and behavioral (Ardouin Scale of Behavior in PD, [5, 6]) clinical scores.
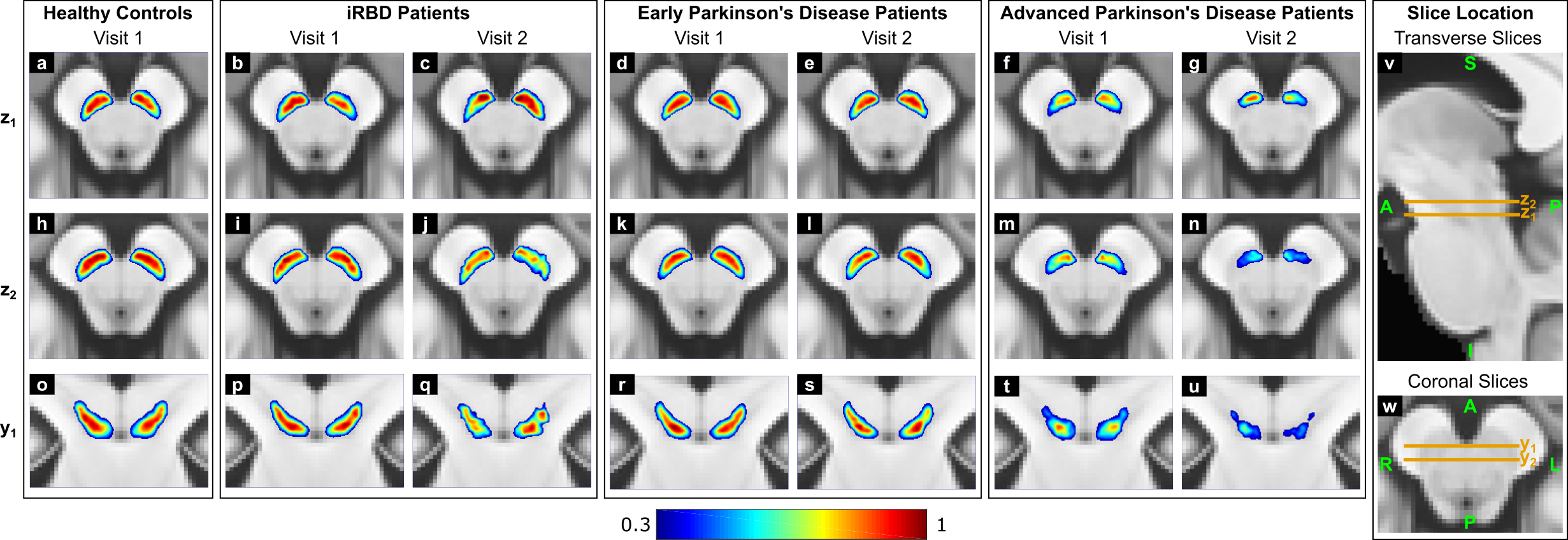
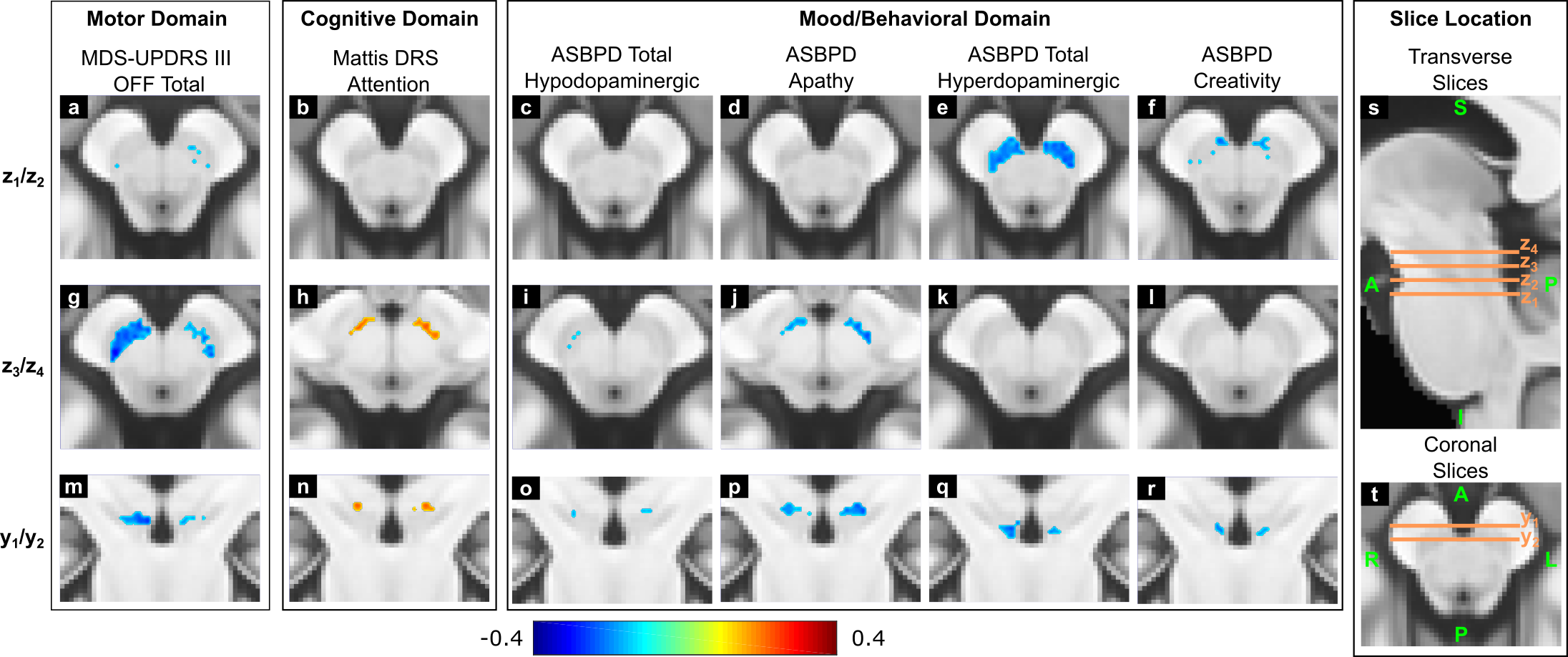
Results: In patients vs. HCs, the SN volume was progressively reduced for increasing disease severity [figure1]. The NM-MRI signal changes appeared to start in the anterolateral motor areas of the SN and then progressed to more medial areas of this region. Voxel-wise patterns of correlation between NM-MRI SNR and motor, cognitive and mood/behavioral clinical scores were localized in distinct regions of the SN [figure2]. This localization reflected the functional organization of the nigrostriatal system observed in histological and electrophysiological studies in non-human primates [7].
Conclusion: NM-MRI enabled assessing voxel-wise longitudinal changes of SN morphology in vivo in humans, including HCs, patients with iRBD or PD, and identifying their correlation with nigral function across all motor, cognitive and behavioral domains. This insight could help assess disease progression in drug trials of disease modification.
References: 1. Sulzer et al. Neuromelanin detection by magnetic resonance imaging (MRI) and its promise as a biomarker for Parkinson’s disease. NPJ Parkinsons Dis 2018; 4: 11. 2. Goetz et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008; 23(15): 2129-70. 3. Mattis et al., Geriatric Psychiatry A Handbook for Psychiatrists and Primary Care Physicians. New York: Grune & Stratton; 1976. 4. Mattis S. Dementia Rating Scale. Professional manual.: Florida: Psychological Assessment Resources; 1988. 5. Ardouin et al. [Assessment of hyper- and hypodopaminergic behaviors in Parkinson’s disease]. Rev Neurol (Paris) 2009; 165(11): 845-56. 6. Rieu et al. International validation of a behavioral scale in Parkinson’s disease without dementia. Mov Disord 2015; 30(5): 705-13. 7. Haber SN. The primate basal ganglia: parallel and integrative networks. Journal of Chemical Neuroanatomy 2003; 26(4): 317-30.
To cite this abstract in AMA style:
E. Biondetti, R. Gaurav, L. Yahia-Cherif, G. Mangone, N. Pyatigorskaya, R. Valabrègue, C. Ewenczyk, M. Hutchison, C. François, J.C Corvol, M. Vidailhet, S. Lehéricy. Spatiotemporal Changes in Substantia Nigra Neuromelanin Content from Prodromal to Clinical Parkinson’s Disease [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/spatiotemporal-changes-in-substantia-nigra-neuromelanin-content-from-prodromal-to-clinical-parkinsons-disease/. Accessed February 2, 2026.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/spatiotemporal-changes-in-substantia-nigra-neuromelanin-content-from-prodromal-to-clinical-parkinsons-disease/