Session Information
Date: Wednesday, September 25, 2019
Session Title: Physiology and Pathophysiology
Session Time: 1:15pm-2:45pm
Location: Les Muses, Level 3
Objective: Profiling gene expression changes in peripheral tissues in PD.
Background: Obtaining tissue for gene expression analysis in PD from CNS is done post mortem. While easily accessible in vivo blood has become popular, it does not seem to yield reproducible changes, therefore, other tissues must be considered. For example the skin, which in PD patients shows symptoms of cutaneous neuropathy, presence of α-synuclein deposits in skin nerve endings and up to fourfold increased risk for melanoma(1-3).
Method: We enrolled 12 + 12 idiopathic PD patients, diagnosed according to the UKPDSBB criteria and on standard antiparkinsonian treatment and 12+12 control subjects for RNA-Seq from skin and blood (results were considered significant at FDR≤0.05). Functional pathway analysis was done using KEGG pathway analysis in R (results were considered significant at FDR≤0,1). Previous work on PD blood with openly accessible changed gene expression lists(4-8) were aggregated using the robust rank method(9) to find most significant changes in single changed gene expression and pathway level.
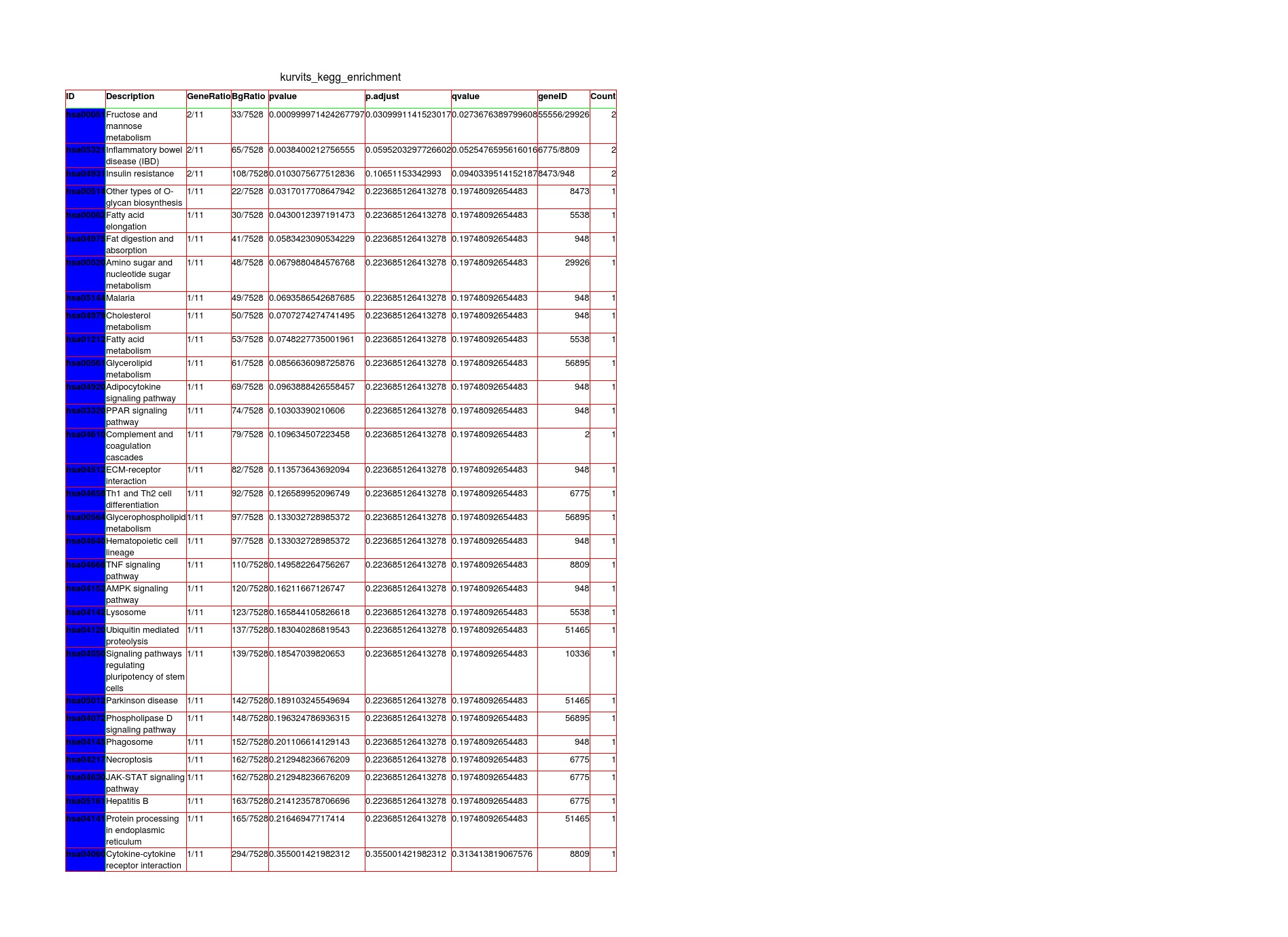
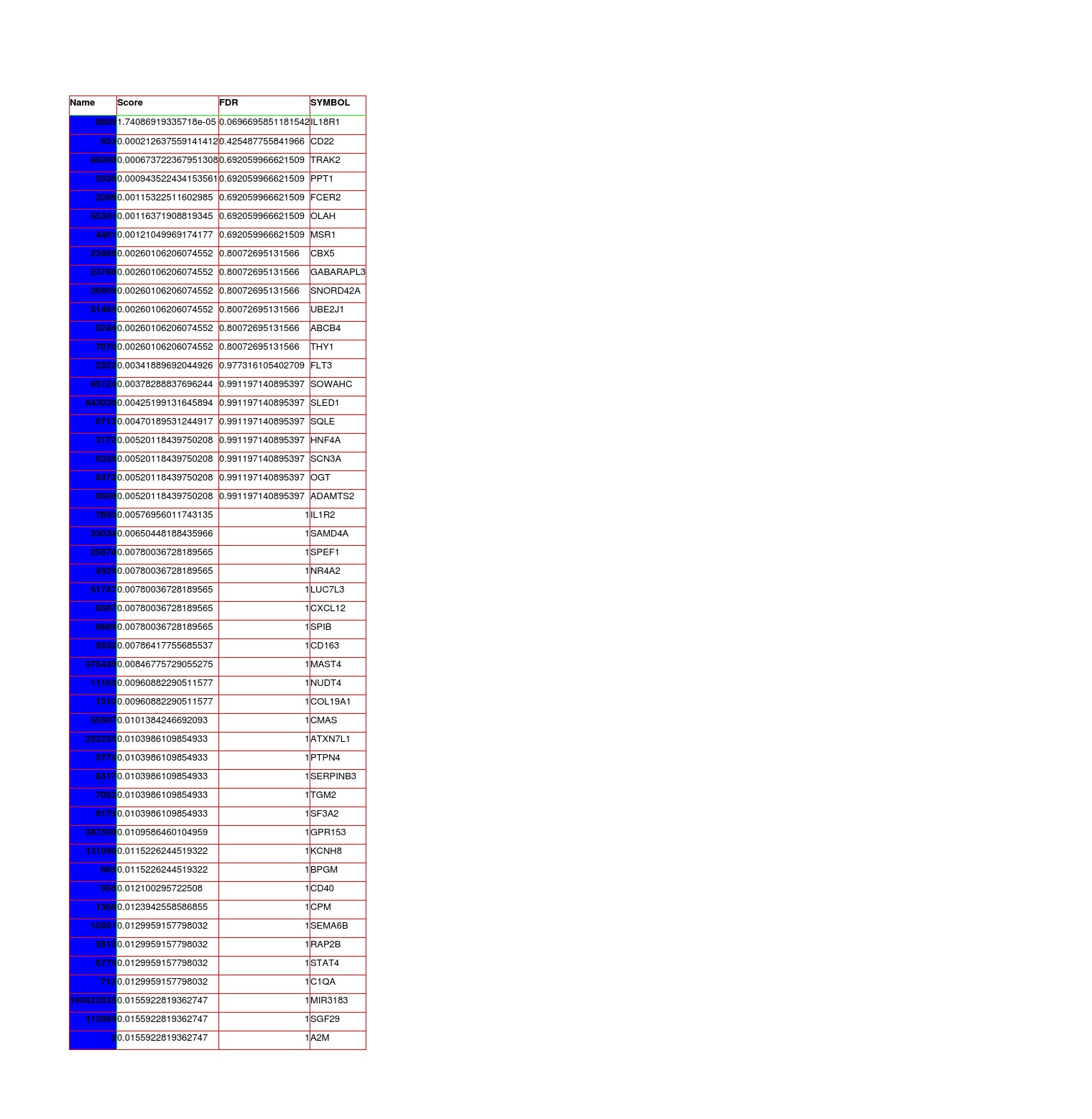
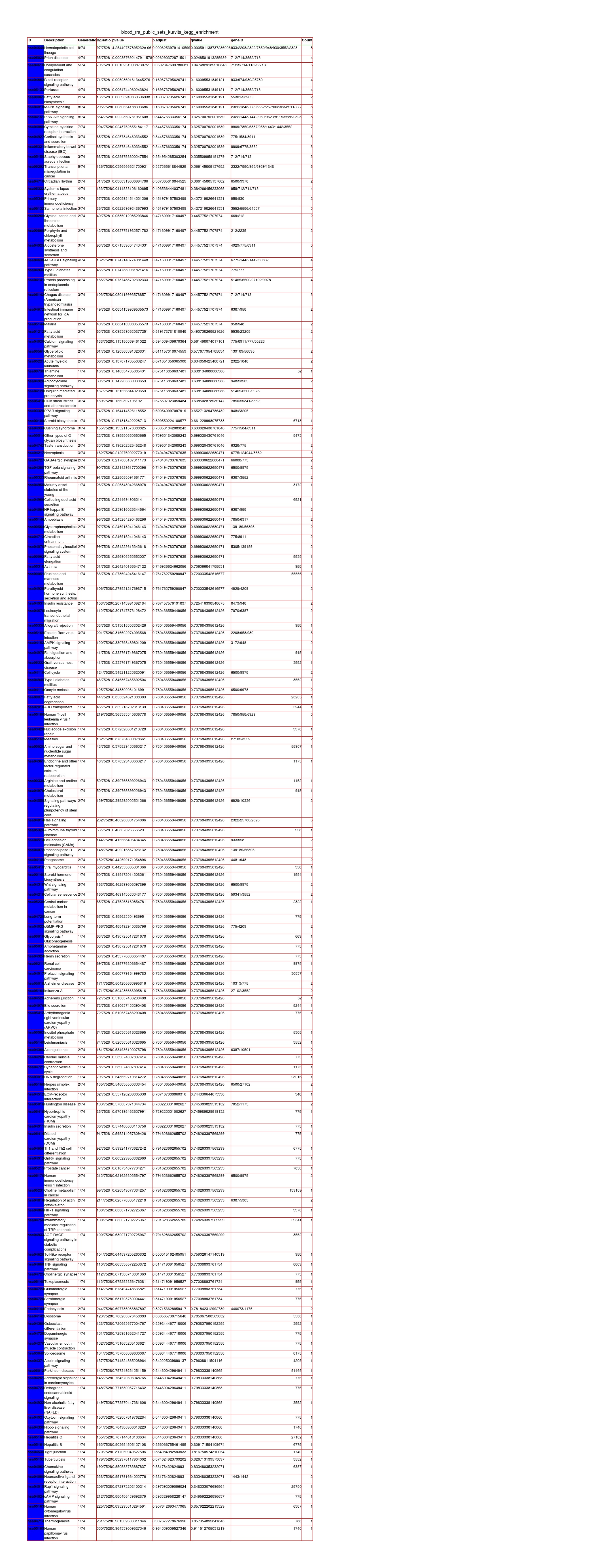
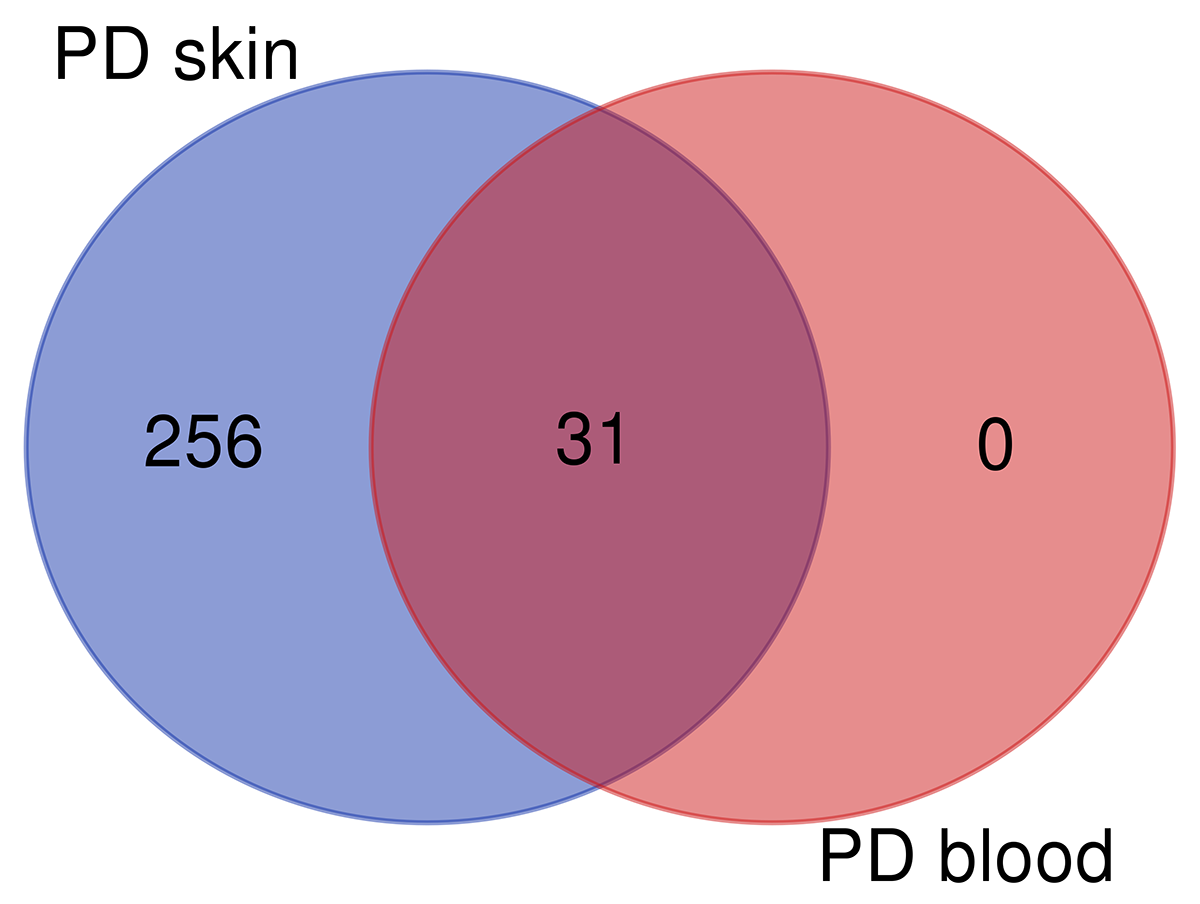
Results: Skin RNA-Seq yielded 1068 differentially expressed genes. A clear pattern of global downregulation appears (874 gene expressions). Top downregulated gene in PD skin is SAA1. Further analysis shows 287 affected pathways in Table 1. [table1] Whole blood RNA-Seq resulted in 26 differentially expressed genes. Most are upregulated but UBE2J1 is downregulated. The 31 affected pathways are listed in Table 2. [table2] Comparing our results from PD blood and 5 other papers, the list of overall highest ranked changed genes is seen in Table 3. [table3] Highest ranked changed pathways in PD blood are seen in Table 4. [table4] Comparison between blood and skin pathways showed an overlap of 100% [figure1] but skin had 256 additional changed pathways related to PD pathology.
Conclusion: Blood and skin yielded different RNA-Seq results with blood being more heterogeneous. Pathway analyses, especially from skin, show concordance with PD pathology in the brain showing that peripheral tissues can be used in PD. Skin has more differentially expressed genes compared to whole blood. The results from blood analyses vary which could be due to the fact that general metabolic and iatrogenic changes affect this tissue more than skin. More studies from PD skin are necessary to assess the reproducibility of the findings and the suitability of skin as a model for PD.
References: 1. Doppler K, Ebert S, Uçeyler N, Trenkwalder C, Ebentheuer J, Volkmann J, et al. Cutaneous neuropathy in Parkinson?s disease: a window into brain pathology. Acta Neuropathol (Berl). 2014 Jul;128(1):99–109. 2. Wang N, Gibbons CH, Lafo J, Freeman R. α-Synuclein in cutaneous autonomic nerves. Neurology. 2013 Oct 29;81(18):1604–10. 3. Liu R, Gao X, Lu Y, Chen H. Meta-analysis of the relationship between Parkinson disease and melanoma. Neurology. 2011 Jun 7;76(23):2002–9. 4. Soreq L, Israel Z, Bergman H, Soreq H. Advanced microarray analysis highlights modified neuro-immune signaling in nucleated blood cells from Parkinson’s disease patients. J Neuroimmunol. 2008 Sep;201–202:227–36. 5. Santiago JA, Potashkin JA. Network-based metaanalysis identifies HNF4A and PTBP1 as longitudinally dynamic biomarkers for Parkinson’s disease. Proc Natl Acad Sci. 2015 Feb 17;112(7):2257–62. 6. Infante J, Prieto C, Sierra M, Sánchez-Juan P, González-Aramburu I, Sánchez-Quintana C, et al. Identification of candidate genes for Parkinson’s disease through blood transcriptome analysis in LRRK2-G2019S carriers, idiopathic cases, and controls. Neurobiol Aging. 2015 Feb;36(2):1105–9. 7. Kedmi M, Bar-Shira A, Gurevich T, Giladi N, Orr-Urtreger A. Decreased expression of B cell related genes in leukocytes of women with Parkinson’s disease. Mol Neurodegener. 2011;6(1):66. 8. Calligaris R, Banica M, Roncaglia P, Robotti E, Finaurini S, Vlachouli C, et al. Blood transcriptomics of drug-naïve sporadic Parkinson’s disease patients. BMC Genomics. 2015 Oct 28;16:876. 9. Kolde R, Laur S, Adler P, Vilo J. Robust rank aggregation for gene list integration and meta-analysis. Bioinformatics. 2012 Feb 15;28(4):573–80.
To cite this abstract in AMA style:
A. Planken, P. Taba, S. Kõks, KM. Kasterpalu, L. Kadastik-Eerme, F. Lättekivi, L. Kurvits, E. Reimann. Skin could be the new peripheral tissue of interest in Parkinson’s disease transcriptomics [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/skin-could-be-the-new-peripheral-tissue-of-interest-in-parkinsons-disease-transcriptomics/. Accessed December 6, 2025.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/skin-could-be-the-new-peripheral-tissue-of-interest-in-parkinsons-disease-transcriptomics/