Session Information
Date: Monday, September 23, 2019
Session Title: Clinical Trials, Pharmacology and Treatment
Session Time: 1:45pm-3:15pm
Location: Agora 3 West, Level 3
Objective: to analyze the long term efficacy and tolerability of safinamide added as adjunctive therapy in a series of patients with Parkinson´s disease (PD)
Background: safinamide is a selective, reversible monoamine oxidase-B inhibitor with both dopaminergic and glutamatergic properties, approved for the treatment of mid- to late-stage PD with fluctuations as add-on therapy to levodopa (LD) alone or in addition to other PD medications.
Method: 169 PD patients were included in the study (52% males, mean age 72.6 years). Mean disease duration was 11.8 years and mean H&Y staging was 2.5. 53% had dyskinesias and 19% cognitive impairment. Patients were treated with 936.9 ± 472.4 mg mean LD equivalent daily dose, 49% with dopamine agonists, and 14% were on advanced therapies. 52% were taking 3 or more PD medications. Safinamide was added as adjunctive therapy due to fluctuations and/or suboptimal motor control in 129 patients and because of suboptimal motor control associated with dyskinesia in 40 patients. Tolerability and response to treatment were recorded at months 6, 12 and 24.
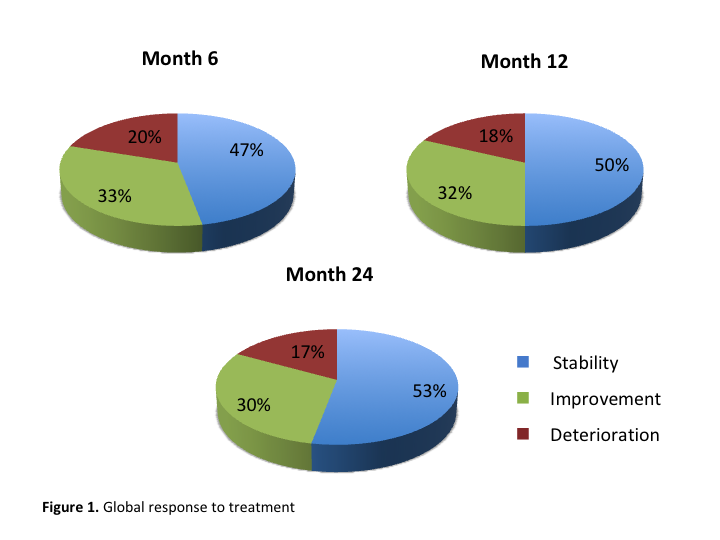
Results: 29% of patients improved during follow up, 50% remained stable, and in 21% the deterioration continued. 54 patients had been on safinamide 24 months or more, and no serious adverse events were recorded. In the whole sample treatment had to be discontinued in 55 patients (32%). The most frequently identified reason for withdrawal was aggravation of dyskinesias (20%), followed by behavioral or cognitive deterioration (13%). When treatment was added in patients with suboptimal motor control associated with dyskinesias withdrawal was more common (55% vs 30%). 44% of the discontinuation of treatment occurred during the first 6 months of drug use. There were no significant differences in demographic or clinical features between the responder group and the discontinuation group.
Conclusion: safinamide has a good tolerability profile in patients with PD despite polytherapy, or advanced state. Most of the patients exhibited a good response to treatment by improving or remaining stable while on safinamide, maintained at 6, 12 and 24 months of follow-up (fig.1). When safinamide was added to control dyskinesia, discontinuation was significantly more common. The incidence of discontinuation in the first months may be partially explained by an initial limited experience using the drug and selecting adequate candidates.
To cite this abstract in AMA style:
A. Vinagre-Aragón, E. Mondragón-Rezola, I. Croitoru, A. Bergareche-Yarza, JJ. Poza-Aldea, A. Olaskoaga-Caballer, M. Iridoy-Zulet, M. Ruibal-Salgado, JC. Gómez-Esteban, K. Berganzo-Corrales, B. Tijero-Merino, J. Ruiz-Martínez. SAFINAMIDE IN DAILY CLINICAL PRACTICE: ASSESSMENT OF EFFICACY AND TOLERABILITY IN PARKINSON´S DISEASE [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/safinamide-in-daily-clinical-practice-assessment-of-efficacy-and-tolerability-in-parkinsons-disease/. Accessed January 18, 2026.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/safinamide-in-daily-clinical-practice-assessment-of-efficacy-and-tolerability-in-parkinsons-disease/