Category: Other
Objective: To assess the safety and efficacy of unilateral FUS subthalamotomy in early PD patients.
Background: FUS subthalamotomy is feasible and therapeutically effective in Parkinson´s disease (PD) [1]
Method: Prospective, open-label study. Eligible patients presented asymmetrical PD with less than 5 years since diagnosis. The primary outcomes were safety and efficacy of motor improvement in the treated hemibody (MDS-UPDRS-III) in the off-medication state at 6 months.
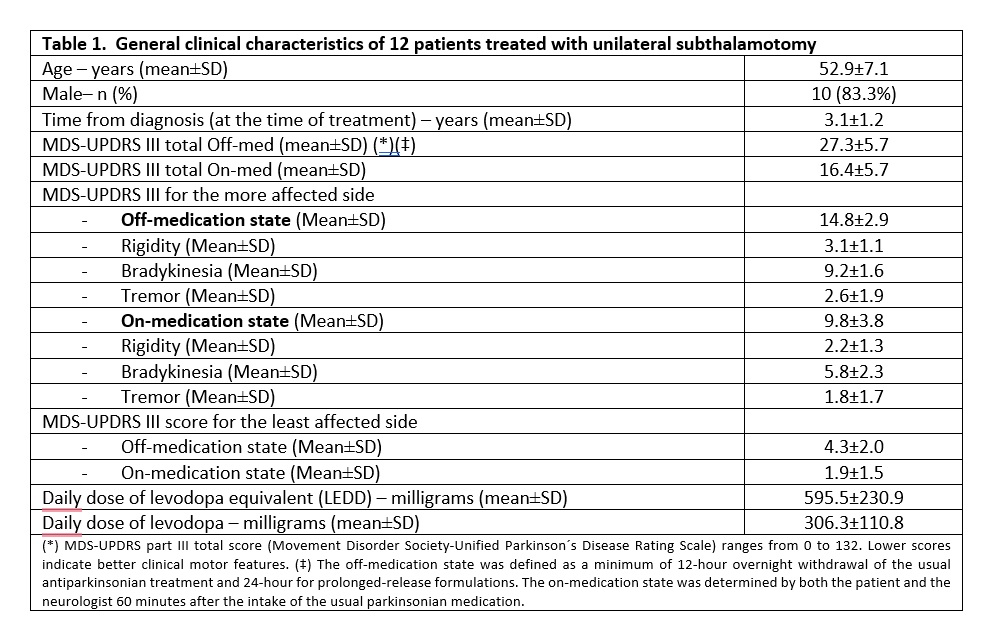
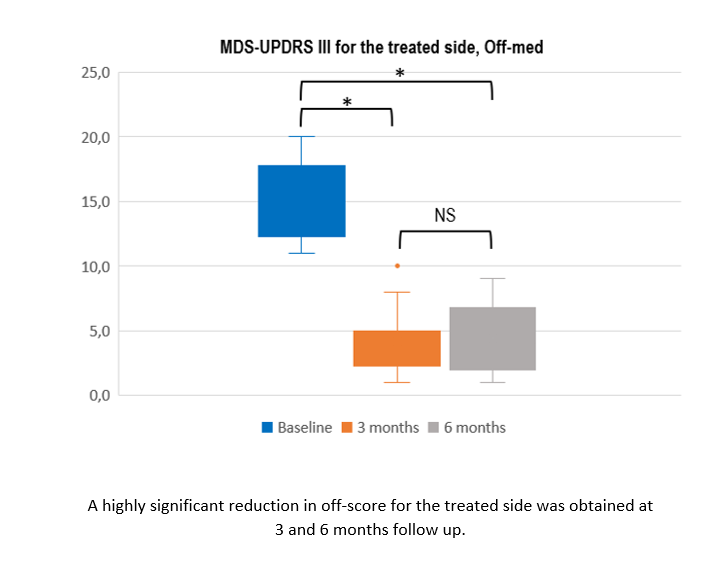
Results: Twelve patients with asymmetrical PD were treated with unilateral FUS-STN. The main clinical features are summarized in table 1. At 6 months, the off-medication MDS-UPDRS III score for the most affected (i.e. treated) side improved by 70.6% (from 14.8±2.9 to 4.4±2.7, p<0.001). Cardinal signs improved by 68.8% for bradykinesia, 66.6% for rigidity, and 90.3% for tremor. The total motor MDS-UPDRS was reduced by 53.4% (from 27.3±5.7 to 13.1±6.1, p<0.001). Within the first 2 weeks after treatment, 2 patients presented mild dyskinesia in the treated side while off-medication and, another 4, in the on-medication state. All cases resolved after levodopa daily dose reduction. At 6 months all were asymptomatic, except one patient reporting occasional and minimal movements in her toes coinciding with levodopa peak of dose-effect. At 6 months follow-up, 1 patient still exhibited facial asymmetry and mildly slurred speech and had also developed off-medication foot and leg dystonia. Six patients exhibited a mean weight increment of 7 Kg that was reduced with diet and exercise. The mean levodopa daily dose decreased from 306.3±110.8 to 187.5±128.1 milligrams (p=0.008). Eleven patients (91.7%) self-reported improvement after treatment. Secondary outcomes like MDS-UPDRS III on-medication, quality of life, non-motor symptoms, and daily activities did change and also presented low scores post-treatment.
Conclusion: Unilateral FUS-STN also has a favorable therapeutic profile in early PD. A controlled trial is warranted.
References: [1] Martínez-Fernández R, Máñez-Miró JU, Rodríguez-Rojas R, Del Álamo M, Shah BB, Hernández-Fernández F, Pineda-Pardo JA, Monje MHG, Fernández-Rodríguez B, Sperling SA, Mata-Marín D, Guida P, Alonso-Frech F, Obeso I, Gasca-Salas C, Vela-Desojo L, Elias WJ, Obeso JA. Randomized Trial of Focused Ultrasound Subthalamotomy for Parkinson’s Disease. N Engl J Med. 2020 Dec 24;383(26):2501-2513. doi: 10.1056/NEJMoa2016311. PMID: 33369354.
To cite this abstract in AMA style:
E. Natera-Villlaba, R. Martínez-Fernández, R. Rodríguez-Rojas, M. Del Alamo, J. Pineda-Pardo, I. Obeso, F. Hernández-Fernández, C. Gasca-Salas, M. Matarazzo, J. Obeso. Safety and Efficacy of unilaterally Focused Ultrasound Subthalamotomy in Early Parkinson´s Disease: The EARLY FOCUS I Study [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/safety-and-efficacy-of-unilaterally-focused-ultrasound-subthalamotomy-in-early-parkinsons-disease-the-early-focus-i-study/. Accessed February 6, 2026.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/safety-and-efficacy-of-unilaterally-focused-ultrasound-subthalamotomy-in-early-parkinsons-disease-the-early-focus-i-study/