Category: Parkinson’s Disease: Clinical Trials
Objective: Evaluate the efficacy of apomorphine (APO) infusion in reducing OFF time during the hours of pump use in PD patients with motor fluctuations inadequately controlled with levodopa and/or adjunctive medications.
Background: APO is a potent, direct and broad spectrum dopaminergic agonist with a rapid onset of action and short clearance half-life. Primary results from this Phase III study confirmed the long-term (1 year) safety and tolerability of APO infusion and showed a mean ±SD reduction of daily OFF time of 3.0 ±3.2 hours per day after 12 weeks maintenance treatment.
Method: This was an open-label, long-term outpatient study of APO infusion (NCT02339064). PD patients with inadequate motor control (≥3 hours of daily OFF time) despite optimized treatment with levodopa and additional PD therapy were eligible for the study. Patients were titrated to individualized APO doses to reach best efficacy without intolerable adverse effects. Once the optimal infusion rate was identified, patients entered a 52-week open-label maintenance period. The pre-specified analysis looked at the improvements over 24 hours. A post-hoc analysis was conducted to examine improvements while the pump was turned on and turned off.
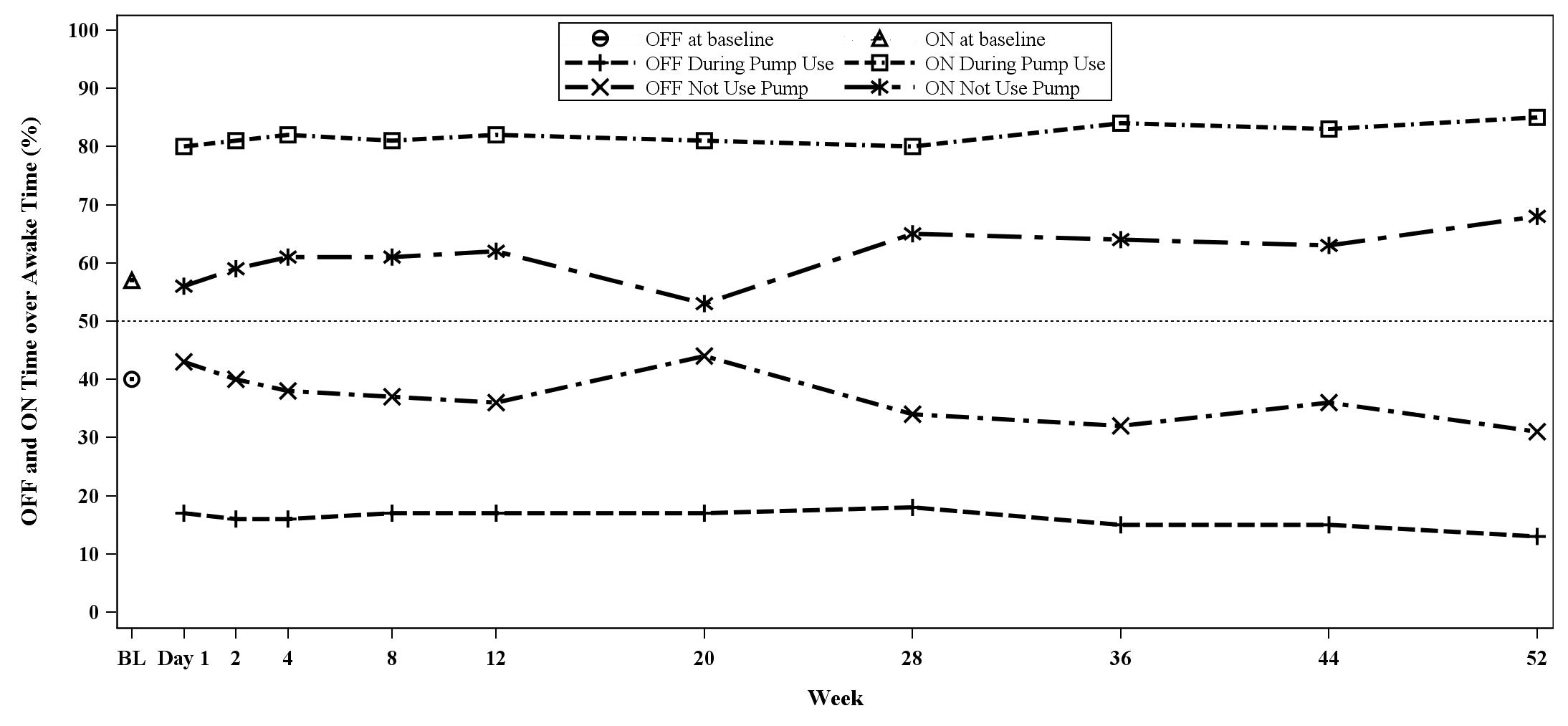
Results: Of the 99 patients enrolled, 85 completed titration, 69 completed 12 weeks maintenance therapy and 46 completed 52 weeks. Following optimal titration, treatment with APO infusion consistently reduced the proportion of awake OFF time from 40% at baseline to <20% when utilizing the pump. Effects were apparent from Day 1 of the maintenance period and were maintained until Week 52 [Figure1]. In contrast, treatment had little effect on awake OFF time when the APO infusion wasn’t used (during the night). Increase ON time without troublesome dyskinesia mirrored OFF time results. Treatment with APO infusion enabled patients to spend ≥80% of their waking day in an ON state without troublesome dyskinesias.
Conclusion: When analyzed during hours of pump use, APO infusion reduced OFF time to <20% of the waking day. Due to its short clearance half-life, there wasn’t carry-over of APO effect during the hours patients removed the pump, and some patients switched to 24-hour use during the study.
To cite this abstract in AMA style:
S. Isaacson, A. Espay, R. Pahwa, T. Clinch, P. LeWitt. Reduction of OFF time with apomorphine infusion during hours of pump use: Analysis from a Phase III, open-label study [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/reduction-of-off-time-with-apomorphine-infusion-during-hours-of-pump-use-analysis-from-a-phase-iii-open-label-study/. Accessed December 22, 2025.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/reduction-of-off-time-with-apomorphine-infusion-during-hours-of-pump-use-analysis-from-a-phase-iii-open-label-study/