Objective: To examine the use of oral anti-parkinsonian medications in advanced Parkinson’s disease (APD) patients who have a history of, or current treatment with deep brain stimulation (DBS) and are receiving carbidopa-levodopa enteral suspension (CLES).
Background: As PD progresses, oral drug regimens may become insufficient for symptom control. Patients using DBS with adjunctive oral therapy may still experience motor fluctuations over time, requiring the addition of therapies like CLES. There is a paucity of real-world data on APD patients with DBS concurrently receiving CLES.
Method: PD-DUAL is a multi-center, retrospective chart review of APD patients with DBS who are treated with CLES in a routine clinical setting in the United States. Included patients had ≥2 recorded visits before CLES initiation and 2 visits (6 months) follow-up after CLES initiation. Estimated enrollment is 50 patients. Primary endpoint: mean decrease from baseline of oral levodopa equivalent daily dose during a 16-hour waking day 6 months after CLES initiation. Secondary endpoints: percentage of individuals with reductions from baseline in total oral levodopa daily dose after CLES initiation, latency from CLES initiation until introduction/tapering of each PD medication, percentage of patients treated with DBS and CLES for whom CLES is monotherapy, and hours of “Off” and “On” time before and after CLES initiation. Data on DBS use in these patients is also being obtained for analysis.
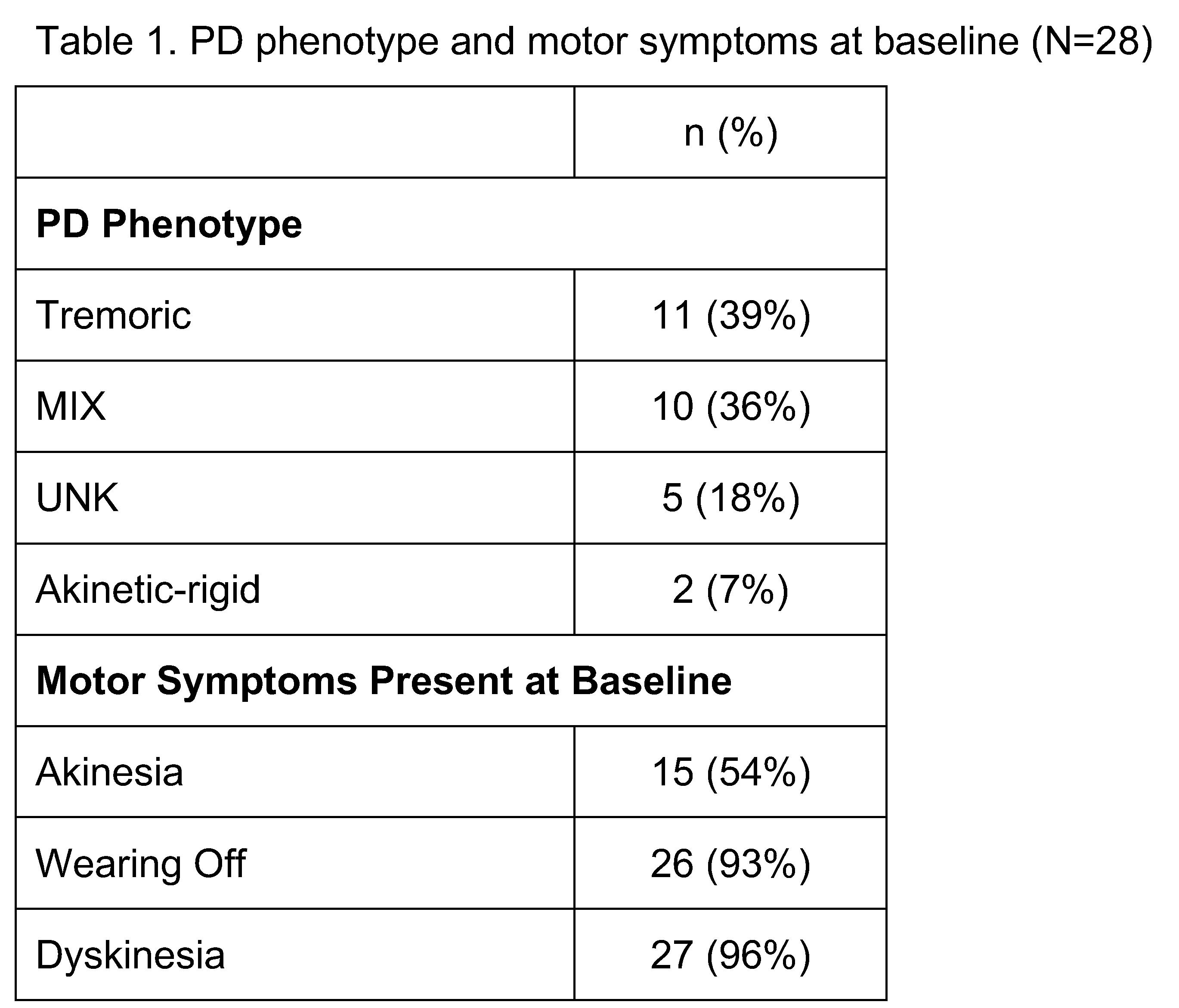
Results: As of 10 February 2020, 31 patients are enrolled from 7 study sites. The mean age is 68.8 ± 5.9 years, 71% are male, 94% are white. Reasons for initiating CLES include (N=28) disabling motor fluctuations/”Off” periods (89%), no longer optimized on concomitant PD medications (71%), uncontrolled dyskinesia (52%), reduced quality of life (32%), refractory dystonia (6.5%), psychiatric effects of DBS (6.5%), cognitive impairment (6.5%), other nonmotor symptoms (6.5%), other reasons (6.5%), and safety reasons related to DBS (3.2%). PD phenotype and motor symptoms at baseline are shown. [table 1] Final results of the study will be presented at the meeting.
Conclusion: Final results of this study will help characterize this APD patient population using both DBS and CLES and provide evidence as to whether concomitant oral medication can be reduced with such treatment.
To cite this abstract in AMA style:
J. Aldred, F. Amjad, A. Johnson, P. Kukreja, I. Malaty, S. Parashos, J. Siddiqui, M. Siddiqui, J. Zamudio, S. Thakkar. Real-World Data on the Use of Levodopa-Carbidopa Intestinal Gel in Patients with Advanced Parkinson’s Disease who are Treated with Deep Brain Stimulation: The PD-DUAL Study [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/real-world-data-on-the-use-of-levodopa-carbidopa-intestinal-gel-in-patients-with-advanced-parkinsons-disease-who-are-treated-with-deep-brain-stimulation-the-pd-dual-study/. Accessed February 7, 2026.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/real-world-data-on-the-use-of-levodopa-carbidopa-intestinal-gel-in-patients-with-advanced-parkinsons-disease-who-are-treated-with-deep-brain-stimulation-the-pd-dual-study/