Session Information
Date: Saturday, October 6, 2018
Session Title: Parkinson’s Disease: Clinical Trials, Pharmacology And Treatment
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
Objective: To examine levodopa-carbidopa intestinal gel, LCIG (also known as carbidopa-levodopa enteral suspension in the US), on non-motor symptoms (NMS) compared with individually-optimized, conventional PD therapies, ie optimized medical treatment (OMT) in advanced Parkinson’s disease (APD).
Background: With conventional therapies, many APD patients experience inadequate motor control and complications. Despite decreased “off” time and increased “on” time without troublesome dyskinesia and reports of NMS improvement, no studies compare the effect of LCIG vs OMT on NMS, including sleep [ref1,2].
Methods: INSIGHTS is a phase 3b, randomized, open-label, multicenter, 26-week study comparing the effect of LCIG vs OMT on NMS in APD (NCT02549092). The study population includes levodopa-responsive APD patients with motor fluctuations no longer controlled by oral PD medications and who experience sleep disturbances as confirmed by a score >18 on the modified Parkinson’s Disease Sleep Scale (PDSS-2). Approximately 88 patients will be enrolled and randomized in a 1:1 ratio to either LCIG or OMT. Primary endpoints include changes from baseline in the Non-Motor Symptoms Scale (NMSS) and the PDSS-2 total scores. Key secondary endpoints measure activities of daily living, quality of life, and safety assessments.
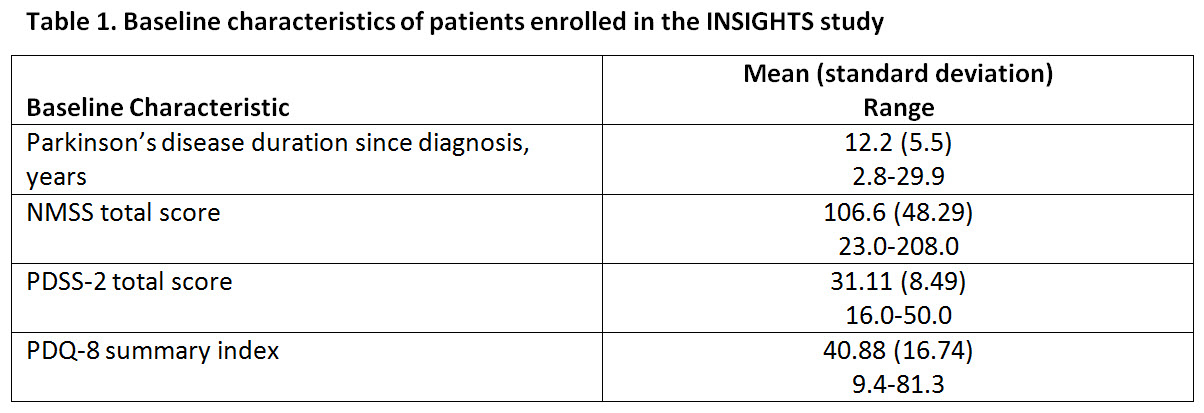
Results: At the current cut-off date, 37 patients have been randomized in the study (Table1). Nearly all patients are white and ≥60 years of age. [table1]
Conclusions: This is the first study comparing the effects of LCIG and OMT on NMS and sleep, and it will provide important information for physicians, patients, and caregivers when assessing the benefits of APD treatment.
References: 1. Fernandez et al. Mov Disord. 2015. 2. Antonini et al. Parkinsonism Relat Disord. 2015.
To cite this abstract in AMA style:
K. Chaudhuri, D. Weintraub, A. Antonini, W. Robieson, M. Li, K. Chatamra, J. Benesh, M. Facheris. Rationale and Design of an Open-Label, Randomized, 26-Week Study Comparing Levodopa-Carbidopa Intestinal Gel to Optimized Medical Treatment on Non-Motor Symptoms in Patients with Advanced Parkinson’s Disease – INSIGHTS Study [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/rationale-and-design-of-an-open-label-randomized-26-week-study-comparing-levodopa-carbidopa-intestinal-gel-to-optimized-medical-treatment-on-non-motor-symptoms-in-patients-with-advanced-parkinson/. Accessed April 20, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/rationale-and-design-of-an-open-label-randomized-26-week-study-comparing-levodopa-carbidopa-intestinal-gel-to-optimized-medical-treatment-on-non-motor-symptoms-in-patients-with-advanced-parkinson/