Session Information
Date: Monday, September 23, 2019
Session Title: Clinical Trials, Pharmacology and Treatment
Session Time: 1:45pm-3:15pm
Location: Agora 3 West, Level 3
Objective: To evaluate the efficacy of buspirone in combination with amantadine in reducing levodopa-induced dyskinesia (LID) in patients with Parkinson’s disease (PD).
Background: LID is an undesirable adverse effect of chronic levodopa (LD) therapy in PD. Approximately half of the patients required medication adjustment or additional medications to manage LID[1]. The increasing utilization of deep brain stimulation for the treatment of LID indicates that best medical management is not enough. Use 5-HT1A agonists as a method of blocking LID in the rat model has opened a door for alternative treatment strategies in humans. Bonifati et al. (1994) found that monotherapy of buspirone 20mg daily had significant reduction in LID (5/7 subjects)[2]. While amantadine is effective at reducing LID, clinical practice has shown that the benefits of the drug are eventually overcome by progression of disease. When a disease becomes refractory to monotherapy adding an adjuvant medication often yields good results.
Method: A randomized, placebo-controlled, double-blind, two-period cross-over study to evaluate the efficacy of a novel treatment combination (amantadine and buspirone) for LID in PD. Each phase titrates study drug (amantadine + placebo or amantadine + buspirone) for two weeks ending in 30 mg/day. Participants complete LID, tremor, and PD ratings during a day-long outpatient visit with 1.5x clinical oral LD dose. Each phase is followed by a 7±5 day washout.
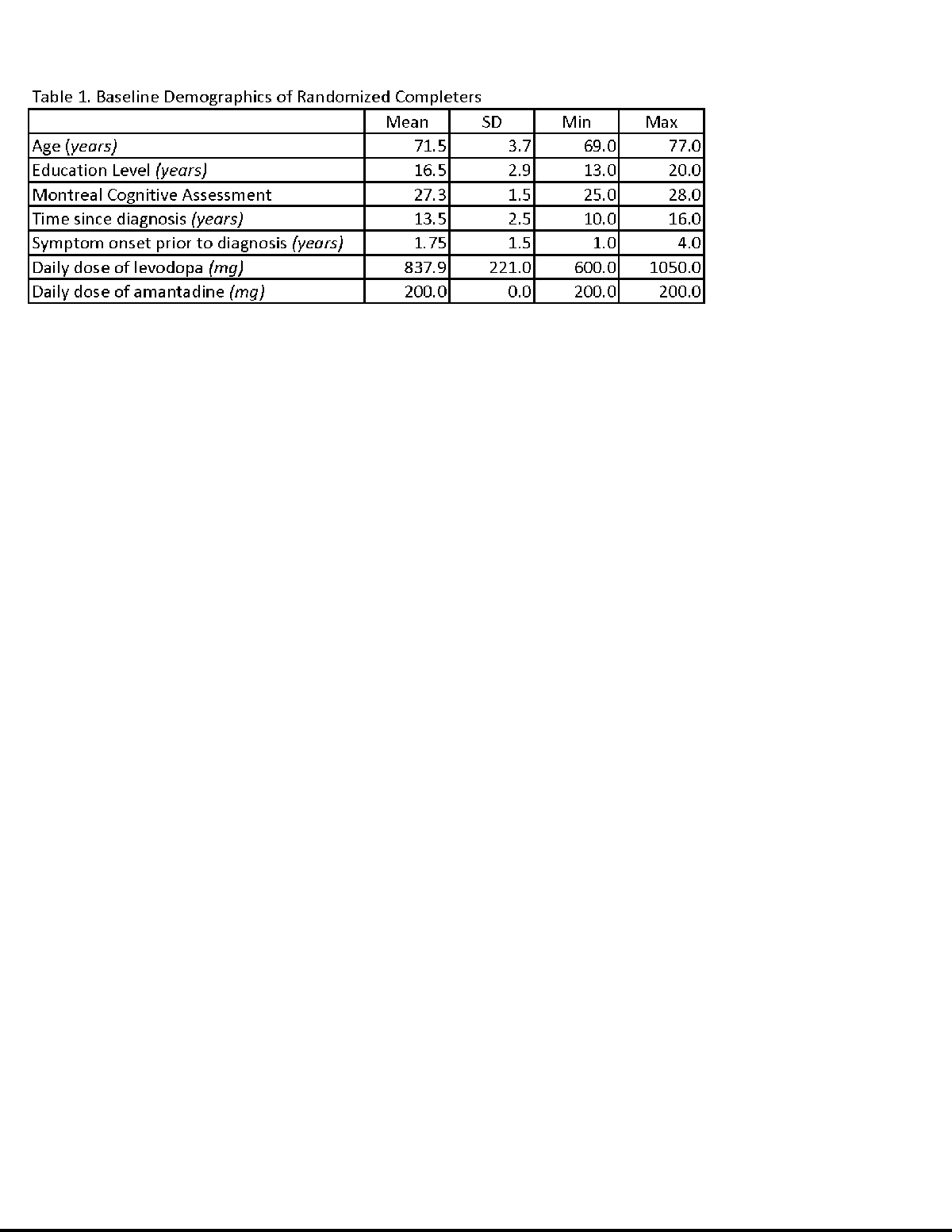
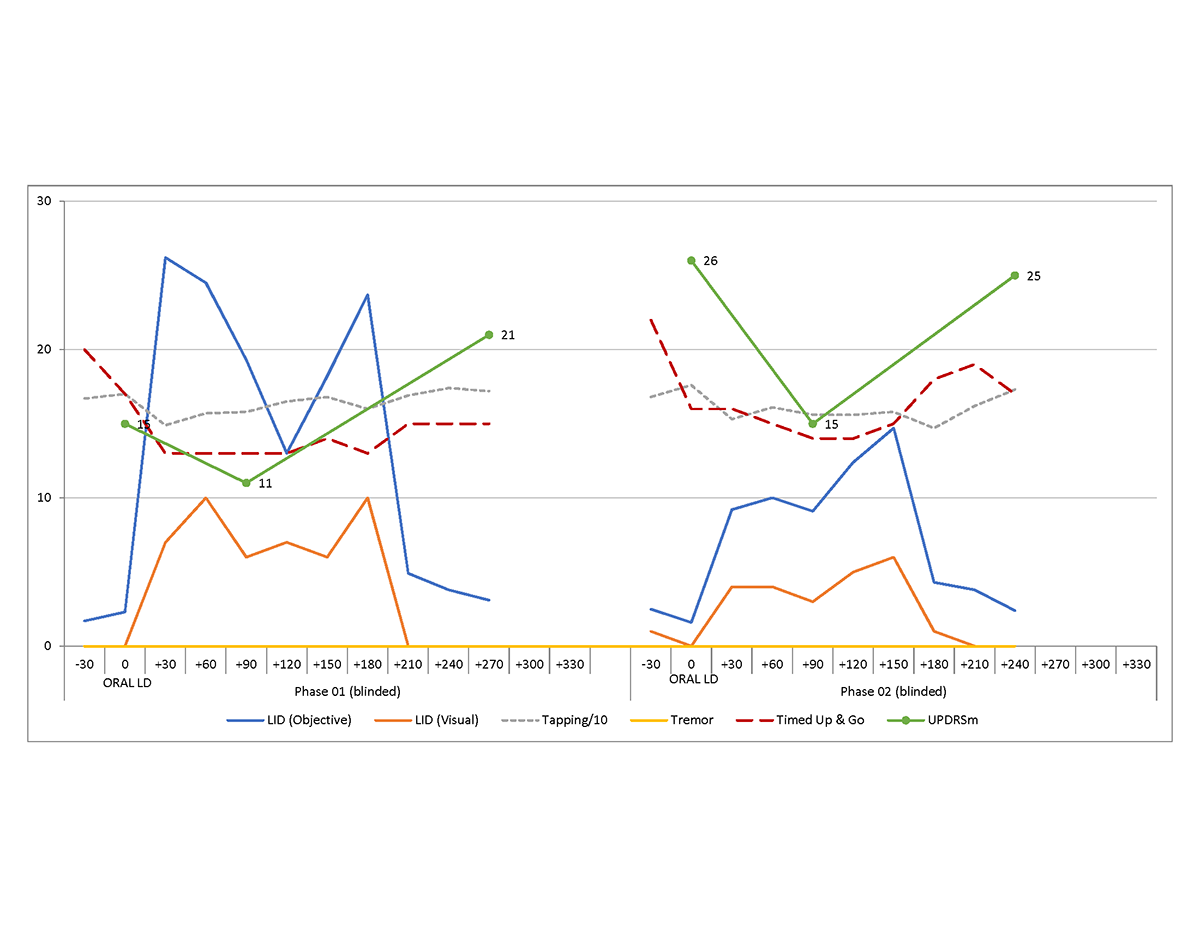
Results: To date, 5 subjects have been enrolled and randomized. One subject withdrew prior to initiating study drug due to difficulties in travelling to the VA for the day-long study visit. All participants were Caucasian non-Hispanic (3 male/1 female). All participants met the United Kingdom Brain Bank criteria for PD. See table 1 for baseline characteristics and figure 1 for an example of the blinded data for one subject over both phases. Analysis is on-going.
Conclusion: With the advanced understanding of the mechanisms by which LID likely occurs, we can now consider adopting the rational polypharmacy approach to its management.
References: [1]. Müller T, Woitalla D, Russ H, Hock K, Haeger DA. Prevalence and treatment strategies of dyskinesia in patients with Parkinson’s disease. Journal of Neural Transmission. 2007;114(8):1023-6. [2]. Bonifati V, Fabrizio E, Cipriani R, Vanacore N, Meco G. Buspirone in levodopa-induced dyskinesias. Clin Neuropharmacol. 1994 Feb;17(1):73-82.
To cite this abstract in AMA style:
K. Chung, B. Lobb, S. O'Connor. Randomized, placebo-controlled, double-blind, two-period cross-over study to evaluate the safety, tolerability, and efficacy of a novel treatment combination for levodopa induced dyskinesia in Parkinson’s Disease (BUS-PD) [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/randomized-placebo-controlled-double-blind-two-period-cross-over-study-to-evaluate-the-safety-tolerability-and-efficacy-of-a-novel-treatment-combination-for-levodopa-induced-dyskinesia-in-parkins/. Accessed December 18, 2025.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/randomized-placebo-controlled-double-blind-two-period-cross-over-study-to-evaluate-the-safety-tolerability-and-efficacy-of-a-novel-treatment-combination-for-levodopa-induced-dyskinesia-in-parkins/