Session Information
Date: Saturday, October 6, 2018
Session Title: Parkinson’s Disease: Clinical Trials, Pharmacology And Treatment
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
Objective: To determine the effectiveness of Adaptive Deep Brain Stimulation (aDBS) in PD patients compared to cDBS and non-medicated PD patients (OFF).
Background: aDBS based on the level of beta oscillatory activity has been proven effective in PD, when clinically assessed in the immediate post-operative phase (Little et al. 2016). However, it is also possible to test aDBS effectiveness in the chronically implanted phase (during battery replacement), circumventing the stun effect of cDBS placement. The advent of validated computer-based tests able to quantify bradykinesia (Homann et al. 2000) opens the possibility to more quantitatively evaluate aDBS in both settings.
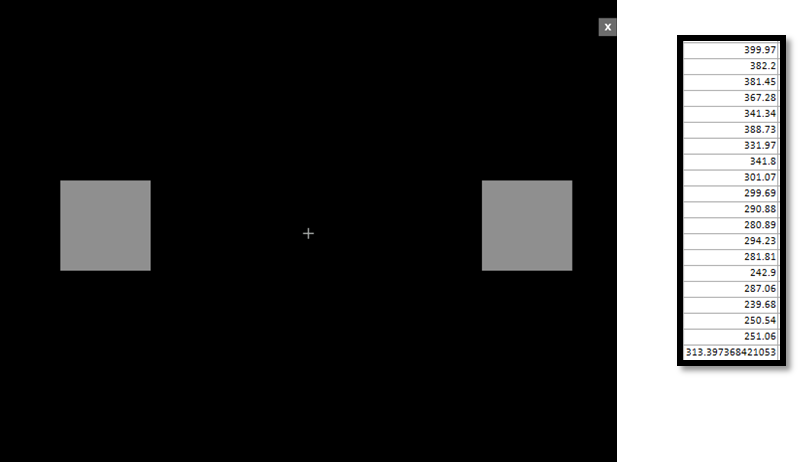
Methods: Thirteen PD patients who underwent either cDBS placement or battery replacement surgery were assessed at baseline (OFF) (ten bilateral, three unilateral) using a modified tabled based version of the bradykinesia akinesia incoordination test (BRAIN test), which consists of 20 trials of alternate finger tapping movements [figure1]. Afterwards, patients were pseudo-randomized in a crossover fashion, starting either with aDBS or cDBS, and assessed two more times, with an interval of ~5 minutes between each condition. Mean dwell times per condition were compared using repeated-measures ANOVA.
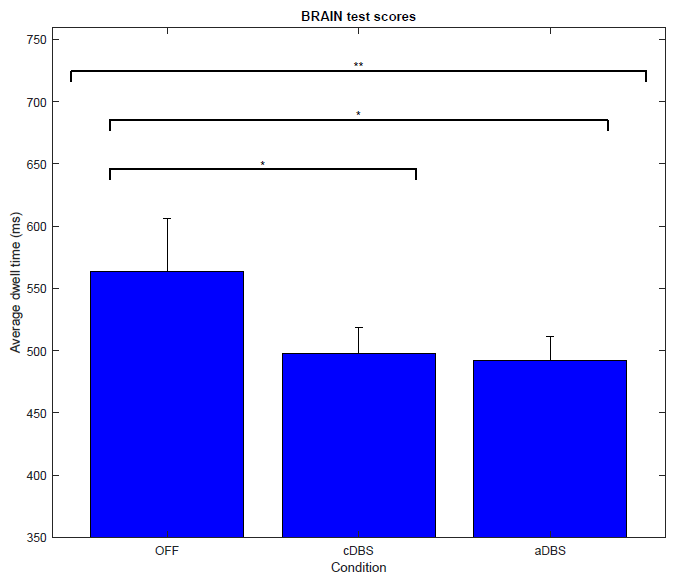
Results: Patients under aDBS (492.1 ± 18.8 ms) and cDBS (497.5 ± 20.7 ms) had significantly lower mean dwell times compared to the OFF state (563.5 ± 42.4 ms) trials. Both types of stimulation showed similar test performance, while patients were stimulated ~40% of the time during aDBS. Main effect: F(1.1, 25.5) = 4.86, p = 0.03**. Simple effects cDBS, p = 0.05* and aDBS, p = 0.02* vs OFF. [figure2].
Conclusions: Quantitatively measured bradykinesia improved after application of both cDBS and aDBS in PD, during acute, as well as chronic implantation phases. This is an additional positive sign favoring a future application of aDBS in PD patients, since aDBS has the added benefit of overall less stimulation. Therefore, less stimulation-related side effects are expected, together with reduced battery consumption and more optimal DBS programming.
References: Homann, CN, K Suppan, K Wenzel, G Giovannoni, G Ivanic, S Horner, E Ott, and HP Hartung. 2000. “The Bradykinesia Akinesia Incoordination Test (BRAIN TEST), an Objective and User-Friendly Means to Evaluate Patients with Parkinsonism.” Movement Disorders : Official Journal of the Movement Disorder Society 15 (4). United States: 641–47. Little, Simon, Martijn Beudel, Ludvic Zrinzo, Thomas Foltynie, Patricia Limousin, Marwan Hariz, Spencer Neal, et al. 2016. “Bilateral Adaptive Deep Brain Stimulation Is Effective in Parkinson’s Disease.” Journal of Neurology, Neurosurgery, and Psychiatry 87 (7). England: 717–21. doi:10.1136/jnnp-2015-310972.
To cite this abstract in AMA style:
D. Piña-Fuentes, M. Tijssen, M. Oterdoom, S. Little, J. van Zijl, T. van Laar, P. Brown, M. van Dijk, M. Beudel. Quantitative assessment of the effectiveness of adaptive Deep Brain Stimulation in Parkinson’s disease [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/quantitative-assessment-of-the-effectiveness-of-adaptive-deep-brain-stimulation-in-parkinsons-disease/. Accessed April 20, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/quantitative-assessment-of-the-effectiveness-of-adaptive-deep-brain-stimulation-in-parkinsons-disease/