Objective: Evaluate the performance of clinical indicators for Device-aided Therapy (DAT) eligibility in real-world setting.
Background: Timely initiation of appropriate DAT may improve quality of life in advanced PD (APD) patients whose symptoms are Inadequately controlled on oral therapy [1]. While consensus criteria for DAT eligibility is emerging [2], there is lack of evidence assessing the real-world performance of those indicators in determining DAT eligibility.
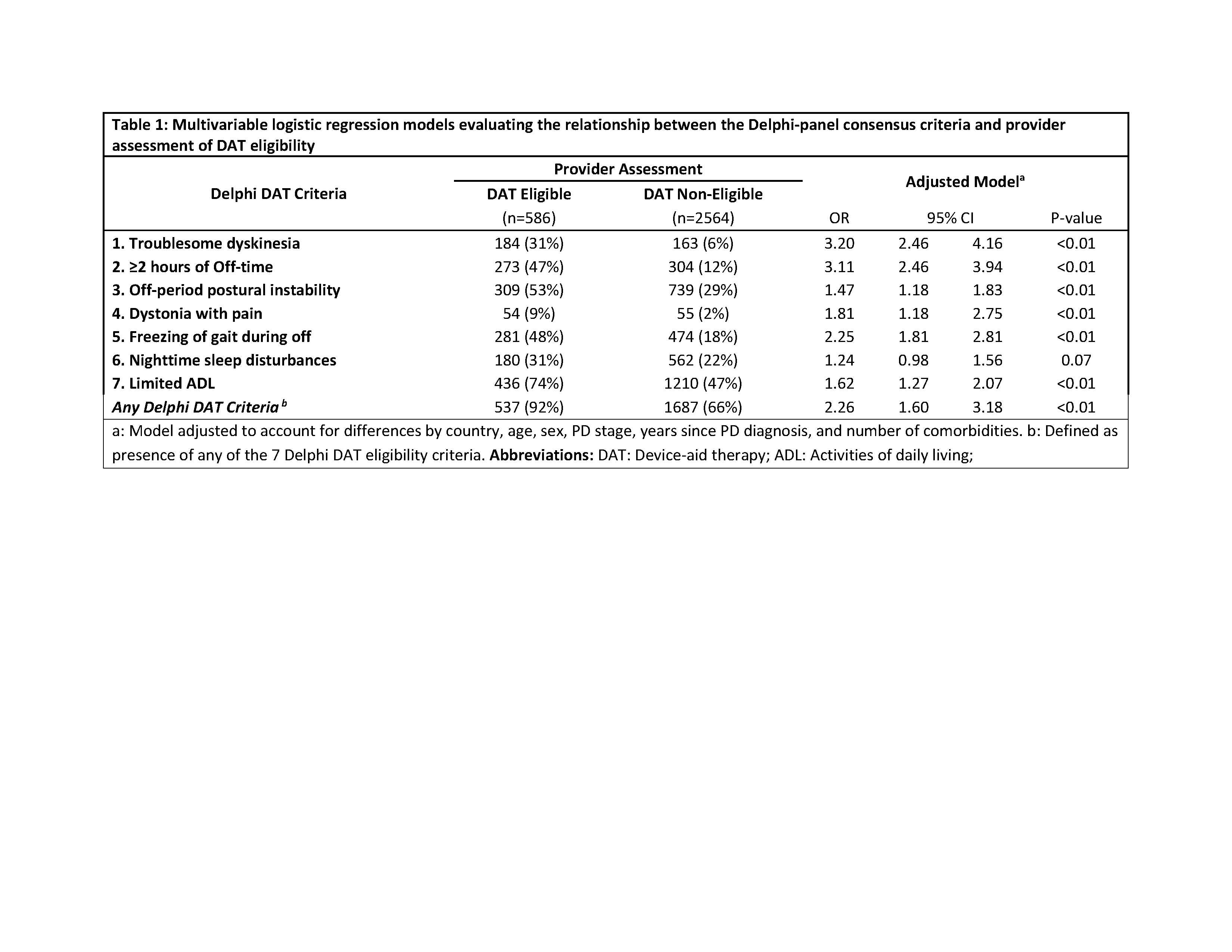
Method: A retrospective analysis from the Adelphi Parkinson’s Disease Specific Programme (2017-2019) dataset was conducted. The sample included patients from 6 countries (US, UK, Germany, France, Italy, or Spain), diagnosed with any PD stage, and were on oral PD therapy. Neurologists’ assessment of DAT eligibility within the next 12-24 months was considered gold standard. DAT eligibility indicators consisted of 7 motor, non-motor and functional symptoms identified by previous Delphi-based consensus study [2]. Multivariable logistic regression models were used to measure the association between neurologist assessment and DAT eligibility indicators. The clinical accuracy of DAT eligibility indicators was compared based on Area Under the Curve (AUC), a measure of accuracy that includes sensitivity and specificity.
Results: In the analytical sample (n=3150), 19% were deemed to be DAT eligible by the neurologists. Compared to non-eligibles, DAT eligible patients had 2.5x higher 12-month hospitalizations (19% vs. 7%, p<0.01) and increased overall caregiver use (54% vs. 31%, p<0.01). Amongst the DAT eligible patients, about 92% patients had at least one of the consensus clinical indicators for DAT. Patients having any of the consensus clinical indicators were more than twice likely (OR: 2.26; 95% CI: 1.60, 3.18) to be considered as a DAT candidate in the next 12-24 months [Table 1]. All the 7 DAT indicators demonstrated high diagnostic accuracy (AUC ≥0.80).
Conclusion: The Delphi-consensus based clinical criteria demonstrated robust psychometric properties. Inclusion of such criteria in clinical pathways and guidelines may facilitate timely identification and referral of DAT eligible patients. Future research should evaluate the impact of different DATs on improving patient outcomes along with reduction of the healthcare and caregiver burden.
References: 1. Timpka J, Nitu B, Datieva V, Odin P, Antonini A. Device-Aided Treatment Strategies in Advanced Parkinson’s Disease. Int Rev Neurobiol. 2017;132:453–474. 2. Antonini A, Stoessl AJ, Kleinman LS, et al. Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson’s disease: a multi-country Delphi-panel approach. Current Medical Research and Opinion. 2018;34(12):2063-2073.
To cite this abstract in AMA style:
K. Chaudhuri, R. Pahwa, S. Isaacson, A. Merola, A. Alobaidi, P. Kandukuri, J.C Parra, L. Bergmann, E. Jones, A. Antonini. Psychometric properties of clinical indicators of device-aided therapy eligibility among Parkinson’s disease patients: Real-world data from 6 countries [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/psychometric-properties-of-clinical-indicators-of-device-aided-therapy-eligibility-among-parkinsons-disease-patients-real-world-data-from-6-countries/. Accessed December 16, 2025.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/psychometric-properties-of-clinical-indicators-of-device-aided-therapy-eligibility-among-parkinsons-disease-patients-real-world-data-from-6-countries/