Category: Parkinson’s Disease: Clinical Trials
Objective: To assess the preliminary efficacy of bilateral intraputaminal delivery of adeno-associated virus serotype 2 containing glial cell line–derived neurotrophic factor (AAV2-GDNF; AB-1005) for patients with mild or moderate Parkinson’s Disease (PD) with up to 36 months of follow-up
Background: Loss of dopaminergic neurons is a key feature of PD. GDNF is required for development and survival of dopaminergic neurons; thus, introduction of GDNF via gene therapy has the potential to halt or alter progression of PD by preserving existing neurons and preventing further neuronal loss
Method: This phase 1b trial enrolled participants with mild (Movement Disorder Society Unified PD Rating Scale [MDS-UPDRS] Part III OFF score ≤32; <5 years from diagnosis) and moderate (33‒60; ≥4 years from diagnosis) PD. Participants received bilateral intraputaminal infusions of AAV2-GDNF (AB-1005; 3.3E12 vg/mL; ≤1.8 mL per putamen) via an optimized convection-enhanced delivery technique with intraoperative MRI monitoring. Efficacy was assessed using PD-specific clinical rating scales
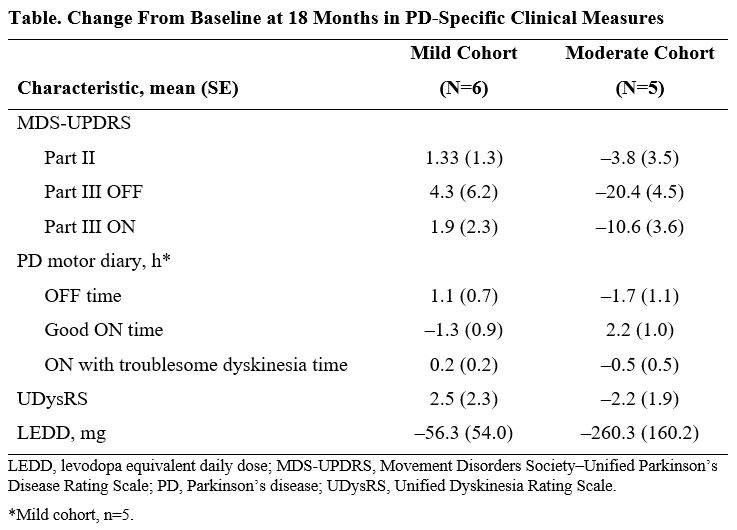
Results: Eleven participants were enrolled (n=6 Mild; n=5 Moderate). Delivery of AAV2-GDNF (AB-1005), covering mean±SE 63%±2% putaminal volume was well tolerated by participants in both cohorts. No adverse events have been attributed to AAV2-GDNF(AB-1005). At 18 months of follow-up, MDS-UPDRS scores, motor diary OFF time, Unified Dyskinesia Rating Scale (UDysRS) scores, and levodopa equivalent daily dose (LEDD) remained stable for patients in the Mild cohort (Table); improvements in mean (±SE) MDS-UPDRS Part III OFF score (–20.4 [±4.5]), motor diary OFF time (–1.70 [±1.10] h), and UDysRS scores (–1.8 [±1.9]) and reductions in LEDD (−260.3 [±160.1] mg) were seen in the Moderate cohort
Conclusion: Patients with mild or moderate PD continued to show stability and/or improvement in PD-specific clinical rating scales at 18 months after AAV2-GDNF (AB-1005) delivery. Data from longer-term follow-up will be available and presented
Table
To cite this abstract in AMA style:
N. Phielipp, C. Christine, A. Merola, J. Elder, P. Larson, W. San Sebastian, M. Fiandaca, C. Urrea, M. Wisniewski, A. van Laar, A. Kells, K. Bankiewicz. Preliminary Efficacy of Bilateral Intraputaminal Delivery of GDNF Gene Therapy (AAV2-GDNF; AB-1005) in Parkinson’s Disease: 18-Month Follow-Up From a Phase 1b Study [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/preliminary-efficacy-of-bilateral-intraputaminal-delivery-of-gdnf-gene-therapy-aav2-gdnf-ab-1005-in-parkinsons-disease-18-month-follow-up-from-a-phase-1b-study/. Accessed April 26, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/preliminary-efficacy-of-bilateral-intraputaminal-delivery-of-gdnf-gene-therapy-aav2-gdnf-ab-1005-in-parkinsons-disease-18-month-follow-up-from-a-phase-1b-study/