Category: Parkinson's Disease: Neuroimaging
Objective: To parcellate the striatum into sub-regions and extract connectivity and surface morphometry features that can distinguish early patients with PD using machine learning.
Background: Parkinson’s disease (PD) is a progressive neurodegenerative disorder with motor and non-motor symptoms, which has no cure or disease-modifying therapies due to the lack of diagnostic biomarkers[1]. Machine learning approaches to distinguish PD have demonstrated high accuracy (over 90%)[2], however, these studies were mostly limited to PD patients with more severe or late-stage PD. It is of greater importance to develop a model that can distinguish PD patients at the earlier stages of disease.
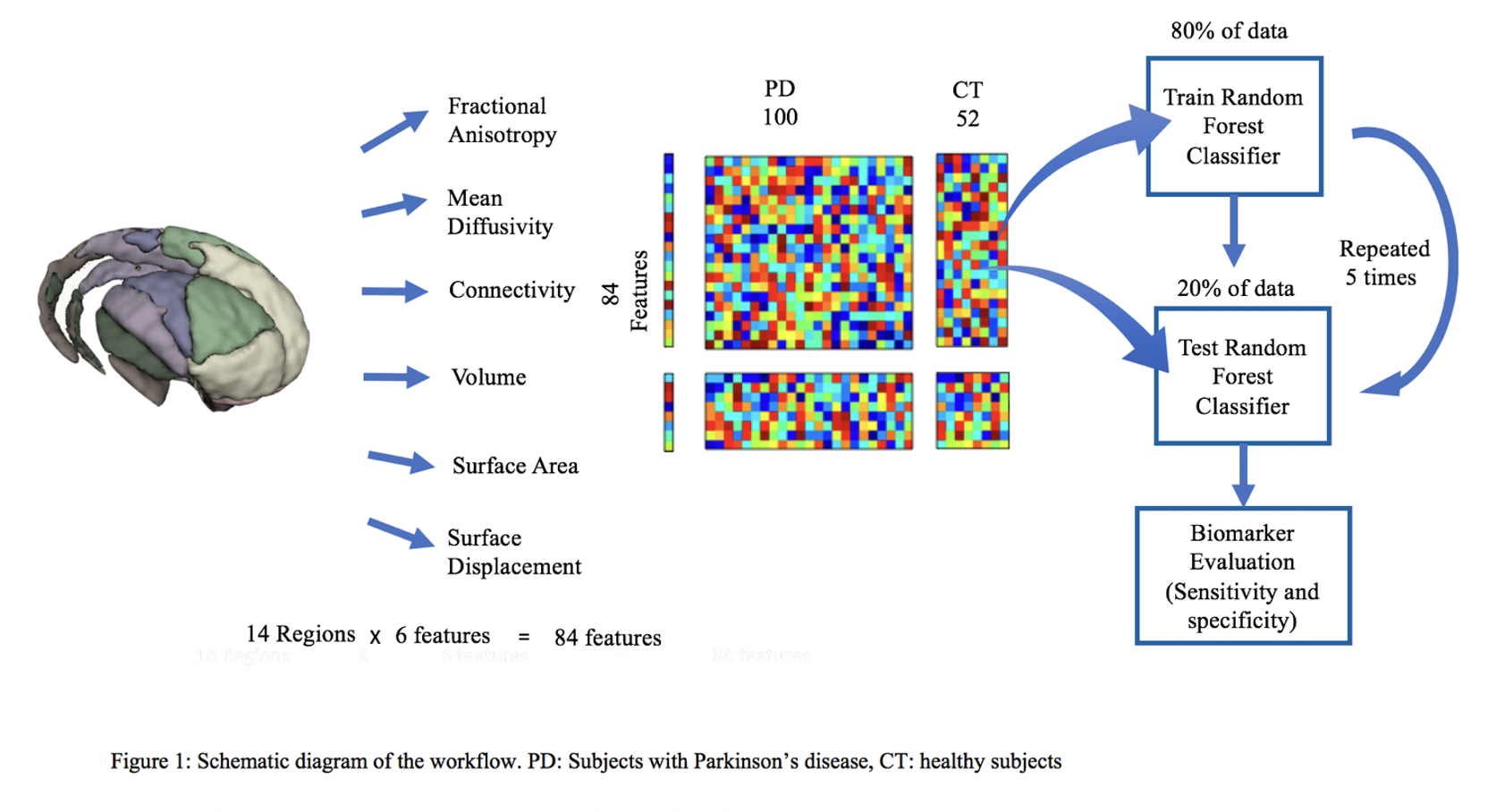
Method: 3T diffusion and T1w MRI data of 100 subjects with PD (unmedicated baseline subjects) and 52 healthy control subjects were obtained from the Parkinson’s Progression Markers Initiative (PPMI)[3] database. Probabilistic tractography[4] was used to parcellate the striatum into 14 sub-regions according to its connectivity to the cortex[5]. Within these sub-regions, features consisted of connected white-matter integrity measures of fractional anisotropy, mean diffusivity, cortical connectivity, surface area and surface displacements [figure1]. A classifier[6] was trained with 80% of the data using these features to distinguish PD subjects from healthy controls. The model was tested on the remaining 20% of the data. An independent dataset of 20 PD and 20 healthy controls (acquired locally) was also used as a separate validation data set. Area under the receiver operating characteristic curves (AUC-ROC) and F1-scores were used to evaluate the accuracies of the models.
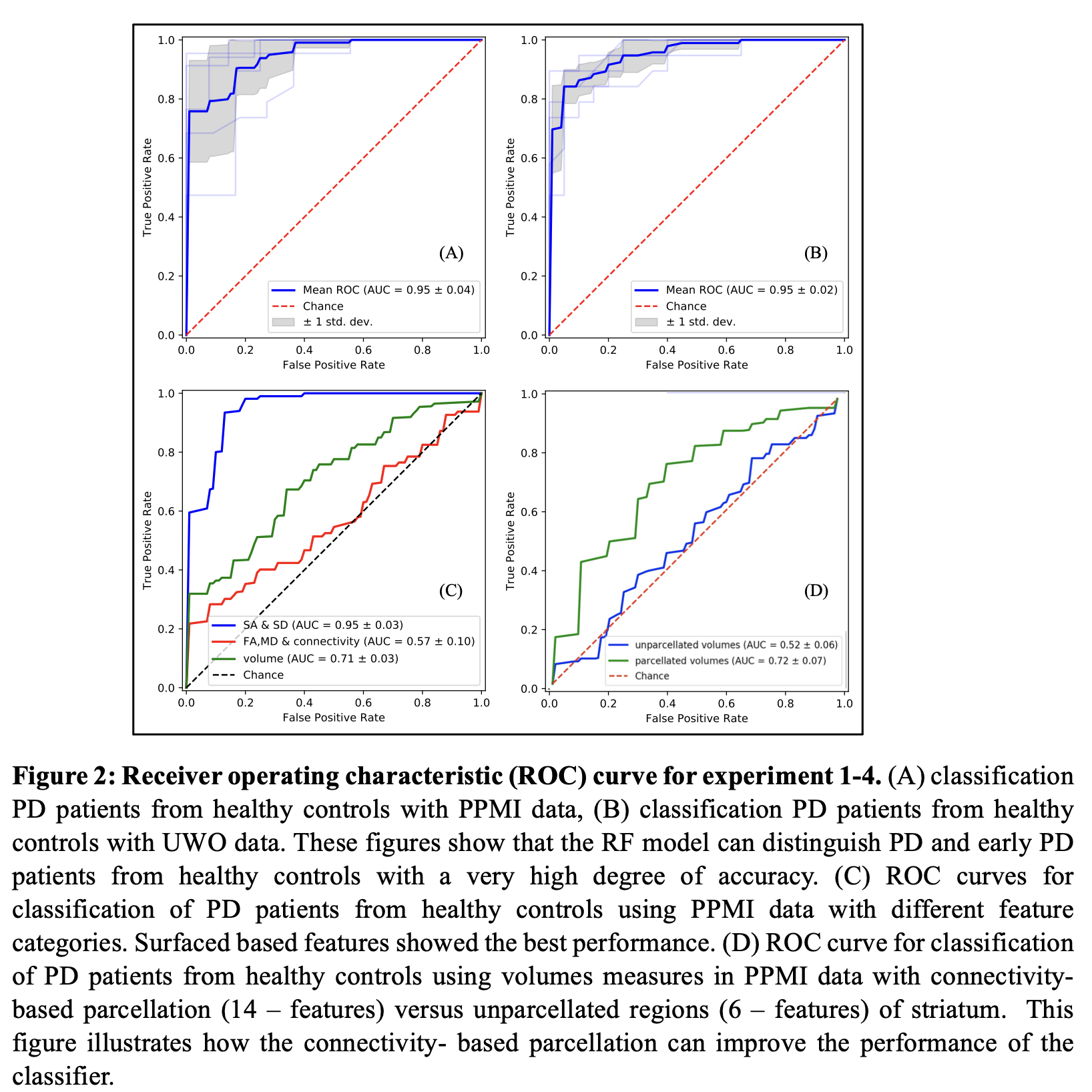
Results: The classifier distinguished PD patients from the healthy controls with an average of 95% AUC-ROC accuracy and F1-score of 88% [figure2]. The validation accuracy with the local dataset was also 95% (F1=89%). Feature importance evaluation shows that surface displacement and surface area features have the highest importance compared to other features in the classifier.
Conclusion: Features extracted from striatal sub-regions distinguished early Parkinson’s patients from healthy controls with 95% accuracy. Surface displacement and surface area features of striatal sub-regions show high importance compared to other features in classifying the two groups, implying that, they can be potential candidates as reliable biomarkers for Parkinson’s disease.
References: [1] D. B. Miller and J. P. O’Callaghan (2015), “Biomarkers of Parkinson’s disease: Present and future,” Metabolism, vol. 64, no. 3, pp. S40–S46.
[2] P. Peran et al., (2018), “MRI supervised and unsupervised classification of Parkinson’s disease and multiple system atrophy,” Mov. Disord., vol. 33, no. 4, pp. 600–608.
[3] K. Marek et al., (2011), “The Parkinson Progression Marker Initiative (PPMI),” Prog. Neurobiol., vol. 95, no. 4, pp. 629–635.
[4] T. E. J. Behrens, H. Johansen Berg, S. Jbabdi, M. F. S. Rushworth, and M. W. Woolrich (2007), “Probabilistic diffusion tractography with multiple fibre orientations: What can we gain?,” Neuroimage, vol. 34, pp. 144–155.
[5] A. C. Tziortzi et al., (2014) “Connectivity-Based Functional Analysis of Dopamine Release in the Striatum Using Diffusion-Weighted MRI and Positron Emission Tomography,” Cereb. Cortex, vol. 24, no. 5, pp. 1165–1177.
[6] L. Breiman (2001), “Random Forests,” Mach. Learn., vol. 45, no. 1, pp. 5–32.
To cite this abstract in AMA style:
D. Henadeerage Don, E. Alushaj, N. Handfield-Jones4, A. Khan, P. Macdonald. Prediction of early-stage Parkinson’s disease using connectivity and morphometry of the striatum [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/prediction-of-early-stage-parkinsons-disease-using-connectivity-and-morphometry-of-the-striatum/. Accessed December 28, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/prediction-of-early-stage-parkinsons-disease-using-connectivity-and-morphometry-of-the-striatum/