Category: Surgical Therapy: Parkinson's Disease
Objective: To predict gait and speech scores of the (MDS)-Unified Parkinson’s Disease Rating Scale (UPDRS) for chronic subthalamic deep brain stimulation (STN-DBS) patients using wearable sensor data.
Background: Chronic STN-DBS patients with speech and gait problems may experience limited axial benefit from high frequency (>100Hz; HF) DBS though studies suggest therapeutic agency from low frequency (60hz; LF) DBS. Though motor benefit is often gauged by clinical rating scales [1], wearable sensor measurements combined with machine learning (ML) methods show promise in predicting PD symptoms [2, 3].
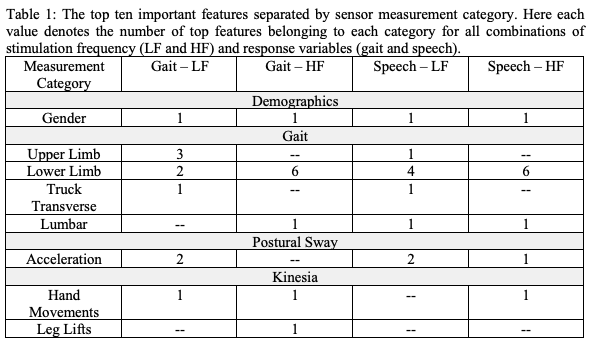
Method: Nine subjects (mean age 65 [57-80]; 7 Males, 2 Females; mean disease duration 15 [5-40] years) with bilateral STN-DBS underwent instrumented gait measurements along with limb bradykinesia and tremor assessments in the medicated state. Each DBS contact pair was tested randomly on both LF (60Hz) and HF (180Hz) at an amplitude (mA) threshold just below the level of side effects. The gait test was conducted with the mobility lab (APDM; OR) during a 7-meter stand and walk test. Tremor and bradykinesia analysis were done with the Kinesia inertial sensors (Great Lakes Technologies, OH). Blinded ratings of speech and gait subscores of MDS-UPDRS III were conducted for each DBS condition. Separate random forest (RF) models [4] were built using sensor data under LF and HF to predict gait and speech subscores. The models were evaluated using leave-one-out cross-validation [5], with one patient in turn excluded from training for testing. The Gini measure of node impurities [6] was used for parameter pruning to the ten most important features.
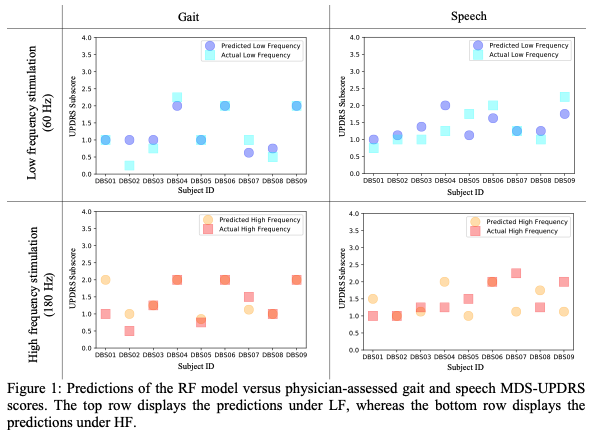
Results: The MDS-UPDRS of all patients for select metrics is presented in Figure 1. The root mean squared error (RMSE) between RF predictions and speech and gait subscores are 0.56 (gait) and 0.65 (speech) for LF and 0.63 (gait) and 0.78 (speech) for HF. Gait parameters (i.e., toe-off angle, cadence, double support cadence, upper and lower limb swing, trunk and lumbar range of motion), hand movement speed, and gender are important features in predicting gait and speech subscores under both LF and HF[Table 1].
Conclusion: The results suggest that RF accurately predicts gait and speech MDS-UPDRS scores using sensor data under both LF and HF STN-DBS—enabling the estimation of clinical ratings in both stimulation conditions.
References: [1] A. Castrioto, A. M. Lozano, Y.-Y. Poon, A. E. Lang, M. Fallis, and E. Moro, “Ten-year outcome of subthalamic stimulation in Parkinson disease: a blinded evaluation,” Archives of neurology, vol. 68, no. 12, pp. 1550-1556, 2011. [2] L. Lonini et al., “Wearable sensors for Parkinson’s disease: which data are worth collecting for training symptom detection models,” NPJ digital medicine, vol. 1, no. 1, pp. 1-8, 2018. [3] E. Rovini, C. Maremmani, and F. Cavallo, “How wearable sensors can support Parkinson’s disease diagnosis and treatment: a systematic review,” Frontiers in neuroscience, vol. 11, p. 555, 2017. [4] L. Breiman, “Random forests,” Machine learning, vol. 45, no. 1, pp. 5-32, 2001. [5] F. Pedregosa et al., “Scikit-learn: machine learning in python,” The Journal of Machine Learning Research, vol. 12, pp. 2825-2830, 2011. [6] P. Tan, “Steinbach M., Kumar V., Introduction to Data Mining,” ed: Boston: Pearson Education, 2006.
To cite this abstract in AMA style:
J. Watts, A. Khojandi, M. Niethammer, R. Ramdhani. Predicting MDS-UPDRS Ratings for Deep Brain Stimulation Patients Using Wearable Sensor Data [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/predicting-mds-updrs-ratings-for-deep-brain-stimulation-patients-using-wearable-sensor-data/. Accessed January 7, 2026.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/predicting-mds-updrs-ratings-for-deep-brain-stimulation-patients-using-wearable-sensor-data/