Category: Parkinson’s Disease: Clinical Trials
Objective: To determine whether digital biomarker sensor data detect slowing in progression of core motor features of Parkinson’s disease (PD) in individuals with early PD treated with prasinezumab.
Background: Prasinezumab was designed to target aggregated alpha-synuclein and slow disease progression in PD. A primary challenge in clinical development is the variability in disease progression measurements, presumably reflecting the fluctuating nature of PD signs and infrequent site visit assessments with different and changing clinical raters. Daily measurement of PD core motor features with digital health technology tools may represent a potential solution to this challenge, enabling frequent and objective measurements.
Method: A total of 316 participants of the Phase II PASADENA Part 1 study (NCT03100149) were randomized 1:1:1 to placebo, prasinezumab 1500 mg and 4500 mg dose groups, whereby pooled dose groups were analyzed here. All received a smartphone/watch, performed daily active motor tests, and carried/wore devices throughout the day. A total of 17 pre-specified sensor features extracted from active test and passive monitoring data were aggregated within participants over fortnights. Linear data were analysed with linear mixed effect models (LMEs) with random slopes fitting each feature’s change from baseline. Mixed Models for Repeated Measurements (MMRM) were used for nonlinear data. Effects of interest were defined as p<0.2, and a 15% false discovery rate was applied.
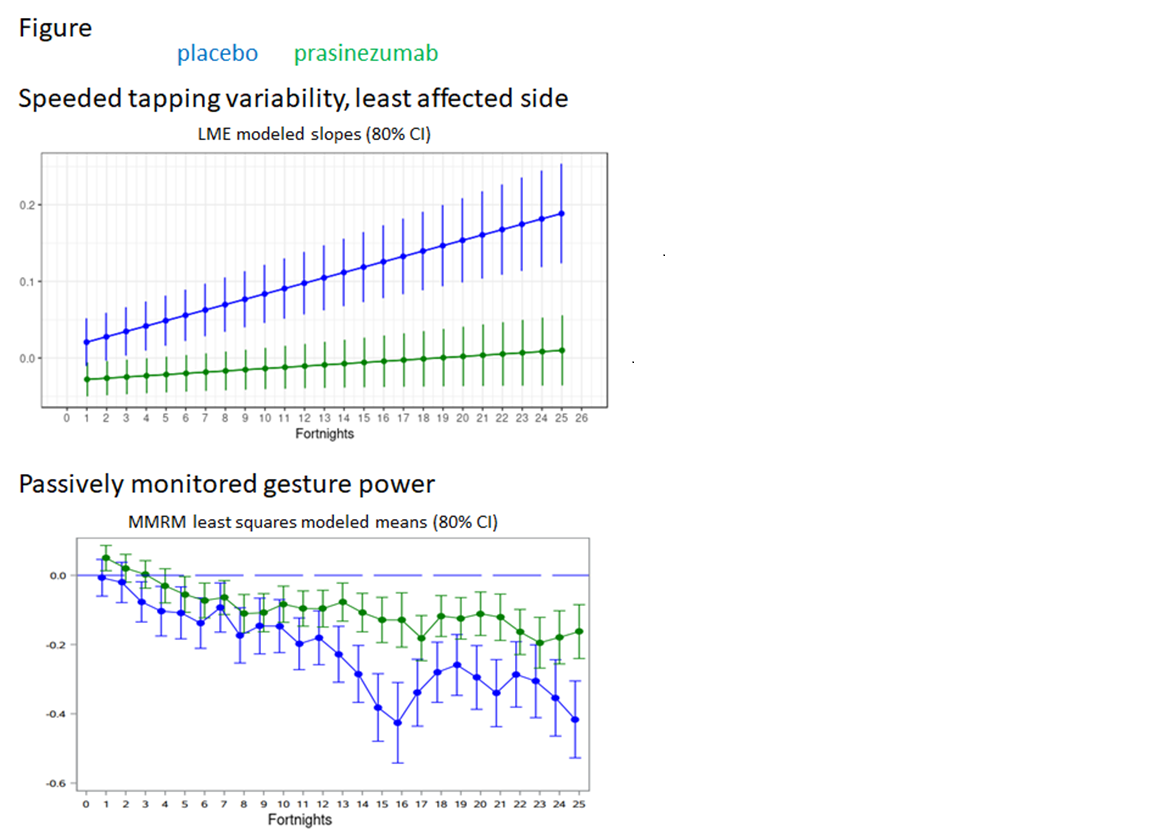
Results: Median adherence was 93.1%, with 75% of patients with mean adherence >75.9%. A total of 5 out of 17 sensor features progressed less in the prasinezumab vs. placebo group: LMEs: speeded tapping variability (each side), U-turn test max. turn speed; MMRMs: maximum pronation/supination speed (most affected side), power of gestures in daily life. Spiral drawing celerity (i.e. accuracy/time), least affected side, showed an effect favoring placebo (LME). Two analyses survived FDR-correction, both favoring prasinezumab: speeded tapping least affected side and gesture power in daily life (see Figure).
Conclusion: The Roche PD Mobile Application v2 demonstrated decreased one-year progression of bradykinesia in prasinezumab vs. placebo treated individuals with early PD, consistent with a disease-modifying effect of prasinezumab.
To cite this abstract in AMA style:
K. Taylor, F. Lipsmeier, E. Volkova-Volkmar, D. Rukina, J. Anzures Cabrera, L. Essioux, M. Abt, B. van Lier, A. Thomann, H. Svoboda, W. Zago, R. Postuma, G. Pagano, M. Lindemann. Prasinezumab reduced progression of Parkinson’s disease motor features measured by Roche PD Mobile Application v2 sensor features: PASADENA Phase II Part 1 [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/prasinezumab-reduced-progression-of-parkinsons-disease-motor-features-measured-by-roche-pd-mobile-application-v2-sensor-features-pasadena-phase-ii-part-1/. Accessed October 21, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/prasinezumab-reduced-progression-of-parkinsons-disease-motor-features-measured-by-roche-pd-mobile-application-v2-sensor-features-pasadena-phase-ii-part-1/