Category: Spasticity
Objective: To examine the pooled incidence of treatment-emergent adverse events (TEAEs) and immunogenicity by indication in sponsored incobotulinumtoxinA (INCO) clinical trials in adults.
Background: The safety and efficacy of INCO in adult neurological disorders has been investigated in multiple prospective clinical trials, including several that are yet to be published. A comprehensive assessment combining available data would provide additional insights into INCO safety.
Method: TEAEs were identified in the integrated clinical database of Merz-sponsored placebo (PBO)-controlled studies in adults with cervical dystonia (CD), blepharospasm, upper limb (UL) and lower limb (LL) spasticity, sialorrhea and essential tremor of the UL. Overall incidences of TEAEs and the categories of serious TEAEs (SAEs), TEAEs leading to discontinuation, fatal TEAEs, TEAEs of special interest (AESIs; indicating possible toxin spread) and treatment-related (TR) events were determined for INCO and PBO after a single injection cycle and for INCO after multiple cycles; the most frequent TEAEs, TR-TEAEs and TR-AESIs after a single cycle of INCO were summarized. Neutralizing antibody (NAb) testing was performed in most studies.
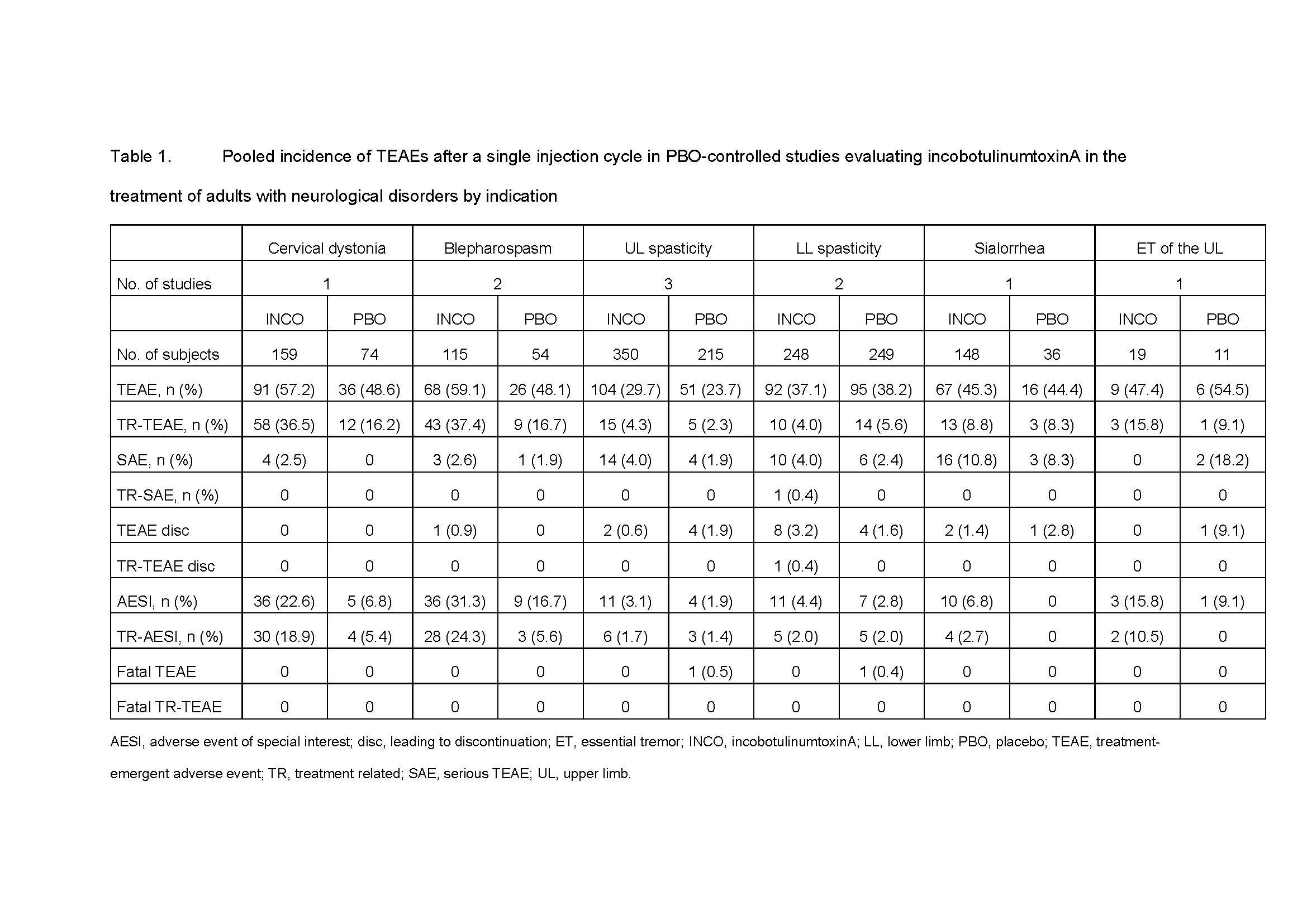
Results: After a single cycle, the incidences of overall TEAEs were similar between INCO and PBO in all indications, although between-indication differences were observed (Table 1). Most TEAEs were mild to moderate in intensity, and only one subject experienced a TR-SAE. Few TEAEs led to discontinuation of INCO; there were no fatal TEAEs in subjects receiving INCO. In general, repeated cycles did not increase the incidences of any TEAE category. The most frequent TEAEs and TR-TEAEs were indication-dependent, but TEAEs usually included nasopharyngitis, diarrhea and headache, and TR-TEAEs generally included dysphagia for indications affecting the head or neck. TR-TEAEs affecting ³1% of patients were uncommon in patients with spasticity. TR-AESIs across all indications were most commonly muscular weakness and dry mouth. Few subjects developed NAb; the vast majority of those positive at the end of the study were also positive at baseline.
Conclusion: Overall, results of this pooled analysis support and extend the favorable safety and tolerability profile of INCO for the treatment of adult neurological disorders established by individual clinical studies.
To cite this abstract in AMA style:
C. Comella, M. Hast, A. Hanschmann. Pooled safety analysis of incobotulinumtoxinA in the treatment of neurological disorders in adults [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/pooled-safety-analysis-of-incobotulinumtoxina-in-the-treatment-of-neurological-disorders-in-adults/. Accessed April 21, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/pooled-safety-analysis-of-incobotulinumtoxina-in-the-treatment-of-neurological-disorders-in-adults/