Session Information
Date: Monday, September 23, 2019
Session Title: Clinical Trials, Pharmacology and Treatment
Session Time: 1:45pm-3:15pm
Location: Agora 3 West, Level 3
Objective: To assess frequency of polyneuropathy in patients with LCIG and the usefulness of routine nerve conduction studies follow-up
Background: Continuous levodopa infusion is an effective treatment for advanced Parkinson’s disease (PD) with motor fluctuations. Polyneuropathy has been reported as a complication in about 10-15 % of patients.
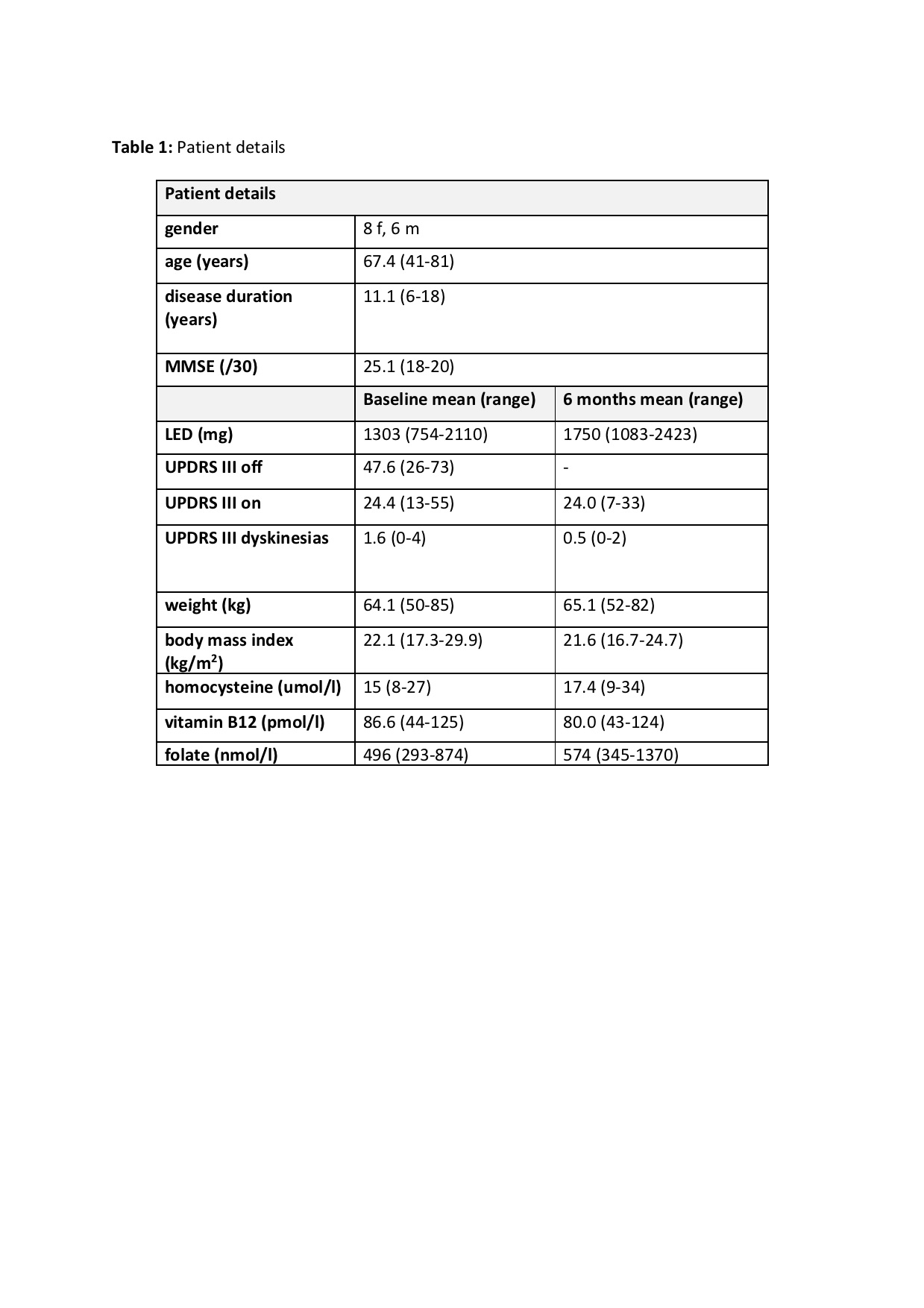
Method: Over a period of 2 years (03/2017 – 02/2019), PD patients started on LCIG infusion were routinely followed including electrophysiological nerve conduction studies at baseline and 6 months after treatment initiation. We prospectively assessed whether patients developed clinical and electrophysiological signs of polyneuropathy. Gender, age, disease duration, mini mental state examination, UPRDS III off at baseline, as well as UPDRS III on, UPDRS III dyskinesia scores, weight, levodopa equivalent dose, plasma levels of homocysteine, folate, vitamin B6 and B12 at baseline and 6 months were also followed.
Results: 14 patients were followed up with at least 2 nerve conduction studies. Patient details can be found in table 1. One out of 14 patients developed electrophysiological signs of sensory axonal polyneuropathy. Two other patients subjectively reported altered sensory perception with a ‘glove and stocking’ distribution, but no significant electrophysiological changes. After substitution of B-vitamins orally, control nerve conduction studies showed improvement. There were no cases of motor axonal or other (demyelinating) polyneuropathies.
Conclusion: In this small cohort of patients, 1/14 patients (7.1 %) developed a sensory axonal neuropathy. The relevance of routine nerve conduction studies in PD patients to detect LCIG-related polyneuropathy remains uncertain. Clinical follow up including sensory symptoms and weight, and laboratory parameters such as homocysteine, vitamin B12 and folate may be useful in identifying at risk patients.
To cite this abstract in AMA style:
KAM. Pauls, J. Toppila, M. Koivu, J. Eerola-Rautio, M. Udd, E. Pekkonen. Polyneuropathy in patients with continuous levodopa/carbidopa intestinal gel (LCIG) infusion [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/polyneuropathy-in-patients-with-continuous-levodopa-carbidopa-intestinal-gel-lcig-infusion/. Accessed April 25, 2025.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/polyneuropathy-in-patients-with-continuous-levodopa-carbidopa-intestinal-gel-lcig-infusion/