Category: Ataxia
Objective: We present the results of plasma biomarker quantification in a cohort of spinocerebellar ataxia type-3 (SCA3) carriers using a Simoa assay.
Background: Development of treatments for SCA3 will require the characterization of accessible and reliable biomarkers that could be used as outcome measures in clinical trials.
Tau, neurofilament light-chain (NFL), glial fibrillary acidic protein (GFAP) and ubiquitin carboxyl-terminal hydrolase like-1 (UCHL-1) have demonstrated a role as blood biomarkers in several neurological disorders[1][2][3][4]. These four analytes can be simultaneously measured using the Neurology 4-Plex A kit in the Simoa platform.
Method: 120 ataxic SCA3 patients, 23 presymptomatic SCA3 carriers and 172 controls were analyzed. Demographic variables, clinical scores (SARA, INAS and ADL) and functional composites (SCAFI, CCFS) were recorded. Plasma samples were analyzed using the Neurology 4-Plex A kit® (Quanterix)[5]. Stata v.15.1 was used to produce multiple linear regression models for adjustment of confounders and detection of effect modification.
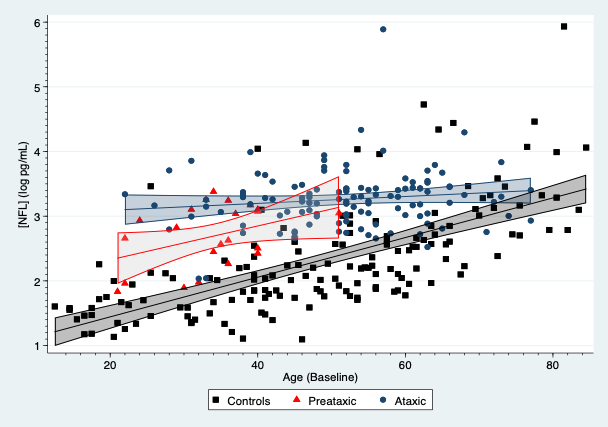
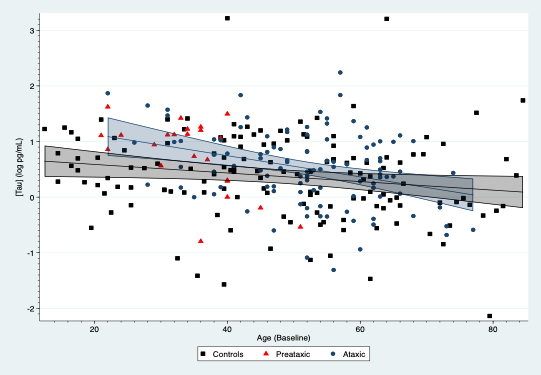
Results: SCA3 patients, presymptomatic carriers and controls yielded differences in their plasmatic NFL concentrations, which were modified by age[figure1]. For subjects aged 30 years, SCA3 patients had an average increase of 1.40 log pg/mL (95%CI: 1.15, 1.65; p<0.001) compared to controls, whereas presymptomatic showed an average rise of 0.83 log pg/mL (95%CI: 0.54, 1.11; p<0.001). NFL concentrations in SCA3 patients were associated with SARA (0.025 log pg/mL per SARA point; 95%CI: 0.012, 0.038) and SCAFI (-0.166 log pg/mL per point in SCAFI; 95%CI: -0.25, -0.08; p<0.001). Age also modified plasmatic tau concentrations, which were raised in mutation carriers, especially symptomatic subjects[figure2]. For subjects aged 30 years, SCA3 patients showed an average increment of 0.42 log pg/mL (95%: 0.10, 0.73; p=0.011) compared to controls. Tau concentrations were associated with the INAS count (0.06 log pg/mL per INAS point; 95%CI: 0.01, 0.10; p=0.012). GFAP and UCHL1 concentrations were similar across the three groups.
Conclusion: NFL and tau might be useful as biomarkers of neurodegeneration in SCA3, since they have shown associations with disease progression scores. Analysis of longitudinal data will allow us to validate these results and clarify their role as potential markers.
Results partially presented in the IARC 2019 on 16/11/19.
References: 1. Byrne LM, Rodrigues FB, Blennow K et al. Neurofilament light protein in blood as a potential biomarker of neurodegeneration in Huntington’s disease: a retrospective cohort analysis. Lancet Neurolol. 2017; 16(8): 601-609. 2. Tatebe H, Kasai T, Ohmichi T. Quantification of plasma phosphorylated tau to use as a biomarker for brain Alzheimer pathology: pilot case-control studies including patients with Alzheimer’s disease and down syndrome. Molecular Neurodegeneration; 2017, 12:63. 3. Posti JP, Takala RS, Runtti H et al. The levels of glial fibrillary acidic protein and ubiquitin C-terminal hydrolase-L1 during the first week after a traumatic brain injury: correlations with clinical and imaging findings. Neurosurgery; 2016. 79:456-464. 4. Zeitlberger AM, Thomas-Black G, Garcia-Moreno H et al. Plasma markers of neurodegeneration are raised in Friedreich’s ataxia. Front Cell Neurosci; 2018, 12:366. 5. Wilson DH, Rissin D, Kan CW et al. The Simoa HD-I analyser: a novel fully Automated Digital Immunoassay analyser with Single-Molecule Sensitivity and Multiplexing. J Lab Autom; 2016, 21:533-547.
To cite this abstract in AMA style:
H. Garcia-Moreno, G. Thomas-Black, A. Heslegrave, H. Zetterberg, P. Giunti. Plasma biomarker quantification in SCA3 using the Neurology 4-PLEX A kit and the Simoa technology [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/plasma-biomarker-quantification-in-sca3-using-the-neurology-4-plex-a-kit-and-the-simoa-technology/. Accessed December 12, 2025.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/plasma-biomarker-quantification-in-sca3-using-the-neurology-4-plex-a-kit-and-the-simoa-technology/