Category: Parkinson’s Disease: Clinical Trials
Objective: To determine whether baseline expression levels for the previously validated sham surgery-related metabolic pattern (SSRP) can be used to predict the magnitude of the placebo response in PD patients under blinded trial conditions.
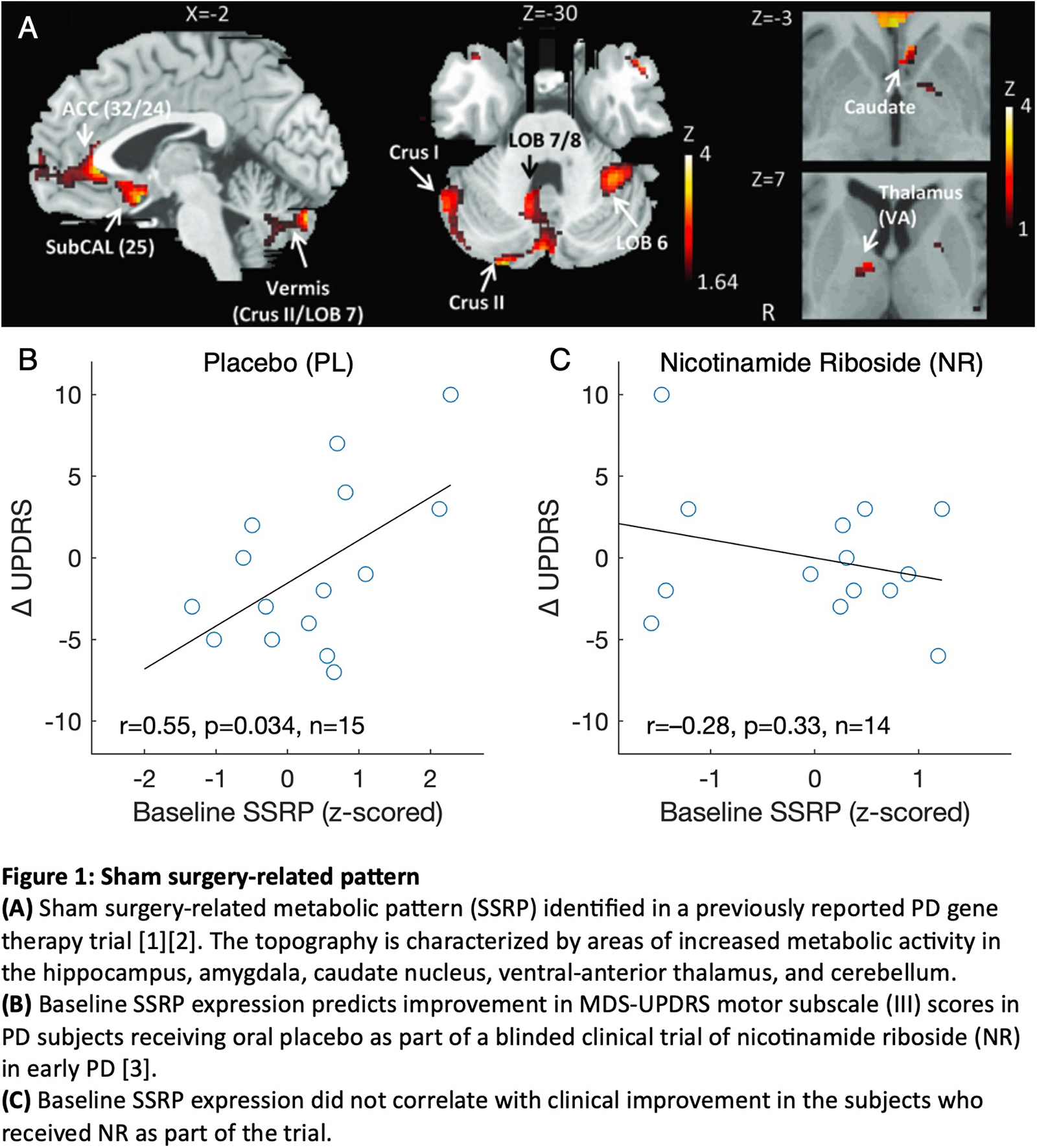
Background: In earlier studies, we have found that sham surgery induced specific effects on brain organization at the network level [1][2]. Analysis of FDG PET data from a randomized, double-blind surgical trial of subthalamic gene therapy for advanced PD revealed a significant sham surgery-related metabolic covariance pattern termed SSRP (Fig 1A). Expression levels for this pattern increased consistently under the blind in the sham surgery group. Motor improvement in these patients was predicted by baseline SSRP expression prior to sham surgery. These data suggest that baseline SSRP expression levels may predict placebo responses in PD clinical trials. However, it is unclear whether this network is generalizable beyond sham surgery.
Method: We analyzed FDG PET data from 29 newly diagnosed, drug-naïve PD patients in a randomized double-blind Phase I trial of nicotinamide riboside (NR) [3]. Scans were obtained at baseline and after 30 days of oral treatment using the 3T Biograph mMR scanner at Haukeland University Hospital (Bergen, Norway). Subjects were evaluated at the time of PET according to the MDS-UPDRS. SSRP expression values were computed at both imaging timepoints and compared across treatment groups. Baseline expression levels and the changes in these values over time were correlated with the corresponding clinical changes in each group. Effects were considered significant for p<0.05, two-tailed.
Results: Baseline SSRP expression in the placebo group correlated with improvement in motor ratings under the blind (PL: p=0.034; Fig 1B); an analogous relationship was not observed in the treatment group (NR: p=0.33; Fig 1C). SSRP changes under the blind, however, did not change significantly in either group (p>0.25), nor did they correlate with concurrent clinical changes (p>0.16).
Conclusion: Although originally identified in a 12 month sham surgery-controlled gene therapy trial in advanced PD, SSRP also predicted the magnitude of the placebo response in an independent 30 day blinded study of an oral supplement administered to de novo patients. Measuring SSRP expression may help identify potential trial participants with increased susceptibility to placebo effects.
References: 1. J. H. Ko et al., “Network modulation following sham surgery in Parkinson’s disease,” J Clin Invest, vol. 124, no. 8, pp. 3656–3666, Aug. 2014, doi: 10.1172/JCI75073.
2. M. Niethammer et al., “Gene therapy reduces Parkinson’s disease symptoms by reorganizing functional brain connectivity,” Sci Transl Med, vol. 10, no. 469, p. eaau0713, Nov. 2018, doi: 10.1126/scitranslmed.aau0713.
3. B. Brakedal et al., “The NADPARK study: A randomized phase I trial of nicotinamide riboside supplementation in Parkinson’s disease,” Cell Metabolism, vol. 34, no. 3, pp. 396-407.e6, Mar. 2022, doi: 10.1016/j.cmet.2022.02.001.
To cite this abstract in AMA style:
J. Barbero, C. Tang, A. Vo, Y. Ma, S. Peng, B. Brakedal, C. Dölle, N. Brekke, C. Tzoulis, D. Eidelberg. Placebo response in Parkinson’s disease (PD) is predicted by expression levels of a specific brain network [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/placebo-response-in-parkinsons-disease-pd-is-predicted-by-expression-levels-of-a-specific-brain-network/. Accessed December 15, 2025.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/placebo-response-in-parkinsons-disease-pd-is-predicted-by-expression-levels-of-a-specific-brain-network/