Category: Surgical Therapy: Parkinson's Disease
Objective: We aimed to assess outcome of GPi-DBS and STN-DBS implanted based on high-resolution MRI.
Background: Both GPi-DBS and STN-DBS are effective for treatment of PD with medically unresponsive fluctuations. There is no definitive consensus on target selection. Generally, STN-DBS allows greater dose reduction of antiparkinsonian drugs and is used more frequently worldwide. GPi-DBS has pronounced direct anti-dyskinetic effect, compromises cognitive and affective state to a lesser extent, and is easier in postoperative programming and management [1,2,3,4,5].
Method: 32 advanced PD-patients were scheduled for receiving GPi-DBS (age at surgery 60.5±8.5 years; PD-duration 13.0±4.3 years). 25 patients received STN-DBS during the same time period (age at surgery 58.0±5.4 years; PD-duration 13.2±3.0 years). Groups shared similar clinical features except for dyskinesia severity (mean UPDRS-4 12.0 versus 10.2, respectively), and MMSE/MoCA score (greater cognitive impairment in GPi-DBS). Preoperative LEDD and L-dopa dose were comparable. Stereotactic planning was performed using high-resolution 3T MRI. We evaluated outcome (UPDRS, ADLs), quality of life (QoL,PDQ-39), antiparkinsonian medication, cognitive and affective state under continuous DBS through 1 year postoperatively.
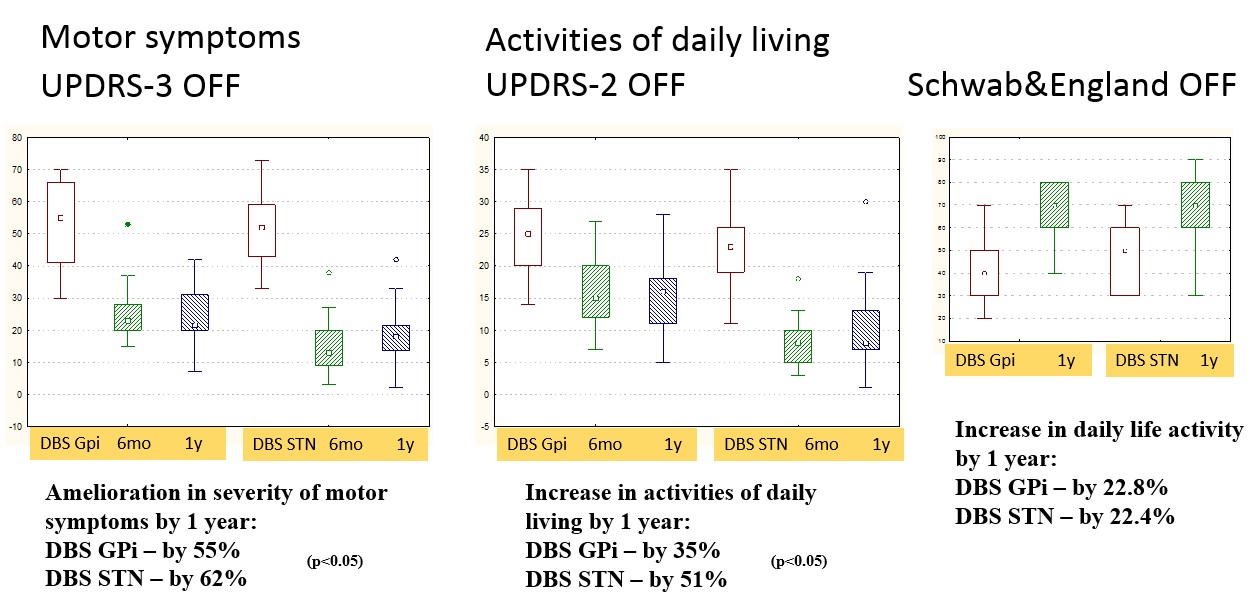
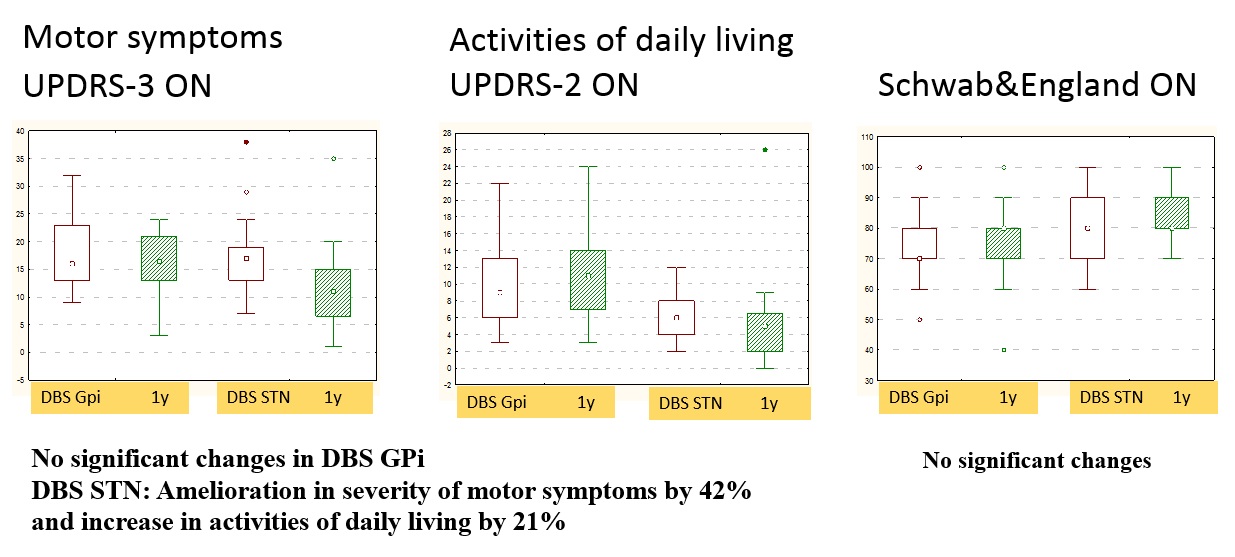
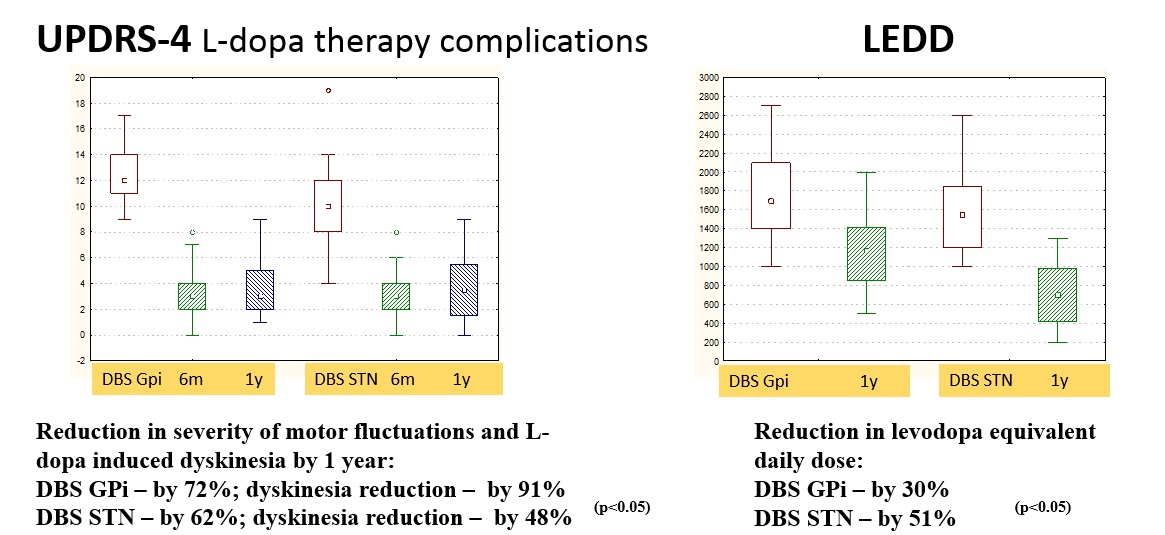
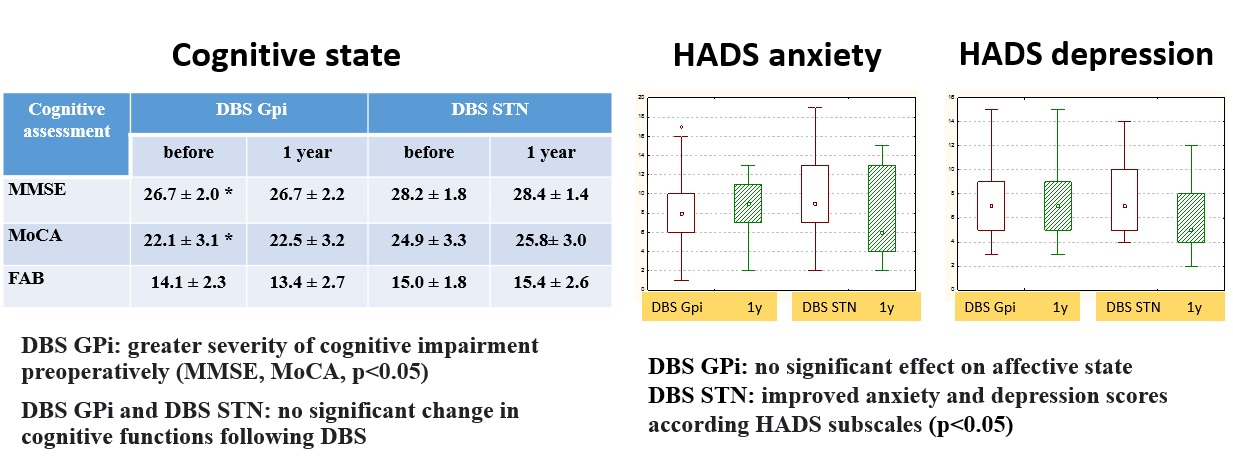
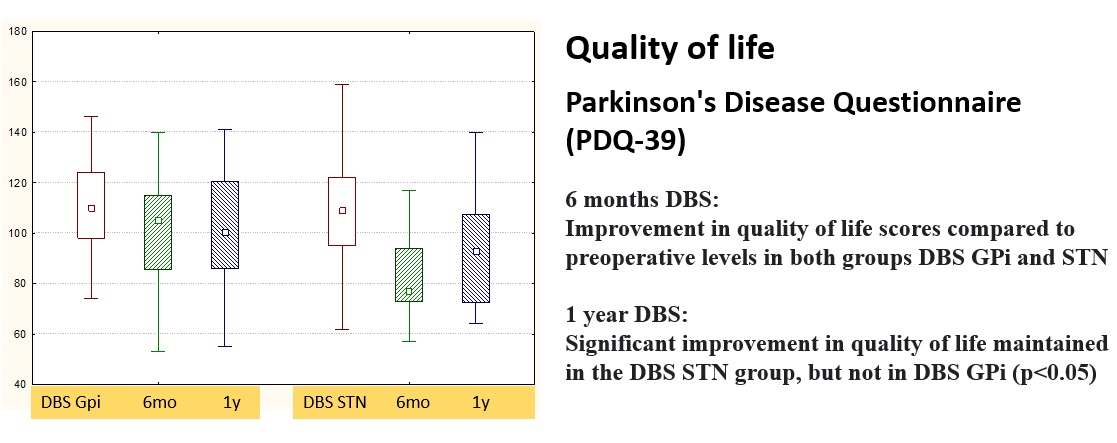
Results: Both targets alleviated significantly PD motor symptoms and ADLs in OFF-state, reduced medication dose, and increased QoL, with superiority for STN-DBS. In GPi-DBS, reduction in UPDRS-3 was 55%, in UPDRS-2 – 35%, in Schwab&England – 23% (OFF), in LEDD – 30% compared to 62, 51, 22, and 51%, respectively, in STN-DBS group. Only STN-DBS ameliorated ON-state quality. GPi-DBS had greater influence on L-dopa therapy complications (dyskinesia). No significant cognitive changes occur in both groups. Psychotic symptoms in early postoperative period were noted more often in STN-DBS (2 vs. 1). Anxiety and depression scores appeared slightly improved in STN-DBS [figure 1,2,3,4,5].
Conclusion: STN-DBS seems to act more comprehensive for PD patients. Despite a greater number of perioperative psychotic reactions, in long-term follow-up STN-DBS has a beneficial effect on affective state and QoL. Nevertheless, GPi-DBS allows performing surgery in patients with poorer cognitive state and severe dyskinesia at low L-dopa doses. When choosing target DBS-structure, a detailed analysis of patient clinical characteristics and individual treatment goals is necessary.
References: 1. Deuschl G, Antonini A, Costa J, Śmiłowska K, Berg D, Corvol JC, Fabbrini G, Ferreira J, Foltynie T, Mir P, Schrag A, Seppi K, Taba P, Ruzicka E, Selikhova M, Henschke N, Villanueva G, Moro E. European Academy of Neurology/Movement Disorder Society-European Section Guideline on the Treatment of Parkinson’s Disease: I. Invasive Therapies. Mov Disord. 2022 Jul;37(7):1360-1374. doi: 10.1002/mds.29066
2. Rughani A, Schwalb JM, Sidiropoulos C, Pilitsis J, Ramirez-Zamora A, Sweet JA, Mittal S, Espay AJ, Martinez JG, Abosch A, Eskandar E, Gross R, Alterman R, Hamani C. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guideline on Subthalamic Nucleus and Globus Pallidus Internus Deep Brain Stimulation for the Treatment of Patients With Parkinson’s Disease: Executive Summary. Neurosurgery. 2018 Jun 1;82(6):753-756. doi: 10.1093/neuros/nyy037
3. Lee PS, Crammond DJ, Richardson RM. Deep Brain Stimulation of the Subthalamic Nucleus and Globus Pallidus for Parkinson’s Disease. Prog Neurol Surg. 2018;33:207-221. doi: 10.1159/000481105
4. Odekerken VJ, van Laar T, Staal MJ, Mosch A, Hoffmann CF, Nijssen PC, Beute GN, van Vugt JP, Lenders MW, Contarino MF, Mink MS, Bour LJ, van den Munckhof P, Schmand BA, de Haan RJ, Schuurman PR, de Bie RM. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): a randomised controlled trial. Lancet Neurol. 2013 Jan;12(1):37-44. doi: 10.1016/S1474-4422(12)70264-8
5. Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P, Marks WJ Jr, Rothlind J, Sagher O, Moy C, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein JM, Stoner G, Starr PA, Simpson R, Baltuch G, De Salles A, Huang GD, Reda DJ; CSP 468 Study Group. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2010 Jun 3;362(22):2077-91. doi: 10.1056/NEJMoa0907083
To cite this abstract in AMA style:
A. Gamaleya, S. Asriants, A. Poddubskaya, A. Dekopov, A. Tomskiy. Pallidal or subthalamic DBS for advanced Parkinson’s disease in the era of high-resolution MRI [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/pallidal-or-subthalamic-dbs-for-advanced-parkinsons-disease-in-the-era-of-high-resolution-mri/. Accessed December 25, 2025.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/pallidal-or-subthalamic-dbs-for-advanced-parkinsons-disease-in-the-era-of-high-resolution-mri/