Category: Dystonia: Clinical Trials and Therapy
Objective: To assess pain reduction in incobotulinumtoxinA (incoA)-treated adults with cervical dystonia (CD)-related moderate or severe pain at baseline using pooled data from four phase 3 and 4 studies.
Background: Pain is a common and disabling symptom of CD. IncoA has shown pain-relieving benefits upon repeated injection in patients with CD-related pain, regardless of pain severity level.
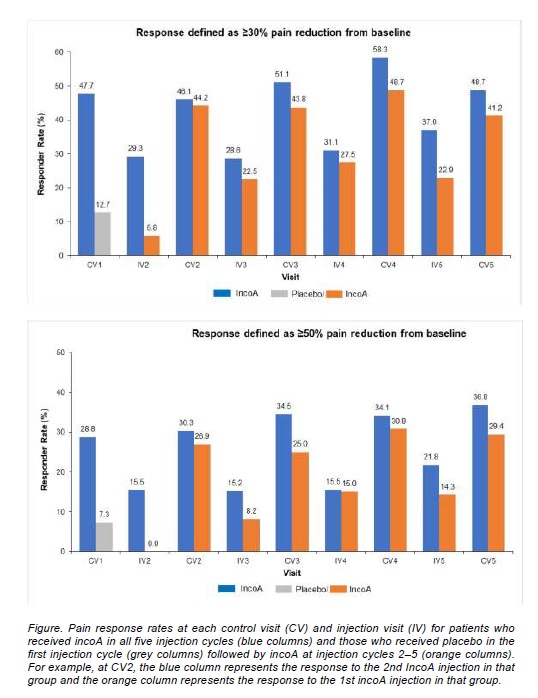
Method: Pooled data from four phase 3 and 4 studies in adults with moderate/severe CD-related pain at baseline were analysed over five injection cycles (IC1‒IC5). One study was placebo-controlled, such that some patients received placebo at IC1 followed by incoA in subsequent ICs (placebo/incoA group). All other patients received incoA at every IC (incoA group). Pain was assessed at each injection visit (IV) and control visit (CV) 4 weeks post-injection using the TWSTRS-pain severity subscale or a pain VAS. Both pain scales were analysed using a score range 0‒10, and pain scores of 3.5‒10 were categorised as moderate/severe. Response was defined as ≥30% or ≥50% reduction in baseline pain score, reflecting at least moderate or substantial clinically relevant improvements, respectively.
Results: Of 451 patients with moderate/severe pain at baseline and pain data at CV1, 396 (87.8%) had received incoA and 55 (12.2%) placebo in IC1. The proportion of these incoA- and placebo-treated patients with ≥30% pain reduction at CV1 were 47.7% and 12.7%, respectively (p<0.0001 Wald-test for difference); the corresponding data for ≥50% pain reduction were 28.8% and 7.3% (p<0.0001 Wald-test for difference). The pain data at CV2‒CV5 for subsequent injection cycles, where all patients received incoA at IV2‒IV5, showed response rates in either group that ranged from 41.2% to 58.3% for ≥30% pain reduction from baseline and from 25.0% to 36.8% for ≥50% pain reduction. Over successive injection cycles, the proportion of incoA-treated patients with pain that had not returned to baseline levels by the next IV tended to increase.
Conclusion: Patients with moderate/severe CD-related pain at baseline experienced clinically important improvements in pain with incoA treatment vs placebo at 4 weeks post-injection. The pain-relieving benefits of incoA were sustained over repeated injection cycles.
To cite this abstract in AMA style:
A. Albanese, J. Wissel, W. Jost, A. Castagna, M. Althaus, G. Comes, A. Scheschonka, M. Vacchelli, H. Jinnah. Pain reduction in adults with cervical dystonia and moderate or severe baseline pain following repeated injections of incobotulinumtoxinA: an analysis of pooled data from phase 3 and 4 studies [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/pain-reduction-in-adults-with-cervical-dystonia-and-moderate-or-severe-baseline-pain-following-repeated-injections-of-incobotulinumtoxina-an-analysis-of-pooled-data-from-phase-3-and-4-studies/. Accessed January 3, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/pain-reduction-in-adults-with-cervical-dystonia-and-moderate-or-severe-baseline-pain-following-repeated-injections-of-incobotulinumtoxina-an-analysis-of-pooled-data-from-phase-3-and-4-studies/