Category: Spasticity
Objective: To assess measures of pain and functional impairment in hemiparetic patients with post-stroke spasticity (PSS) treated with onabotulinumtoxinA (onabotA) from the Adult Spasticity International Registry (ASPIRE) study.
Background: Spasticity is a common occurrence following stroke, and patients with PSS commonly experience functional impairment and pain in upper limbs (UL) and/or lower limbs (LL) that negatively affect quality of life.
Method: ASPIRE (NCT01930786) was a 2-year, international, multicenter, prospective, observational registry study of adult patients with spasticity across multiple etiologies who were treated with onabotA during routine clinical practice. Treatments were given at the clinician’s discretion for 8 total treatment sessions over a 96-week period. This exploratory analysis focuses on hemiparetic patients with spasticity resulting from ischemic, hemorrhagic, or embolic stroke who received treatment for both UL and LL spasticity at the same session for ≥1 session during the study. Patients may have had other etiologies in addition to stroke. Functional impairment was assessed with the Disability Assessment Scale (DAS) and pain levels were assessed with the Numeric Pain Rating Scale (NPRS).
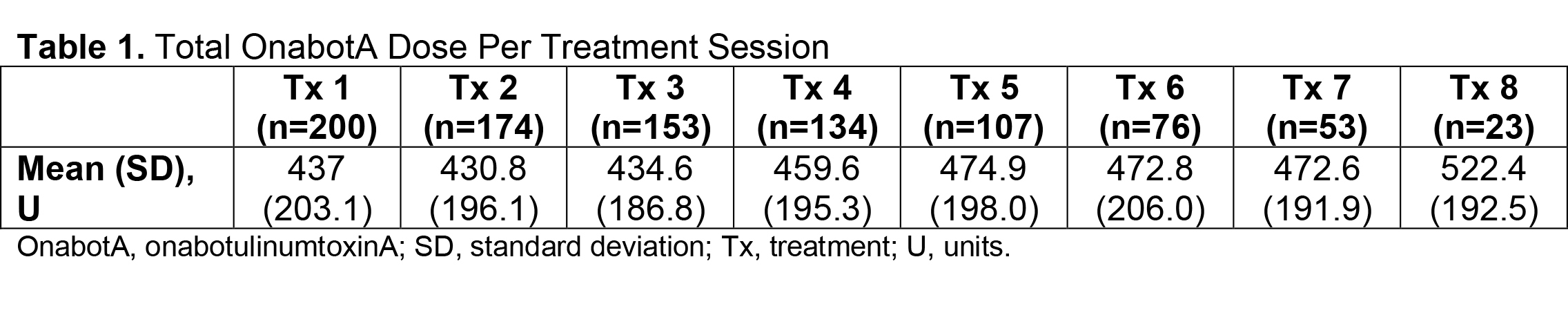
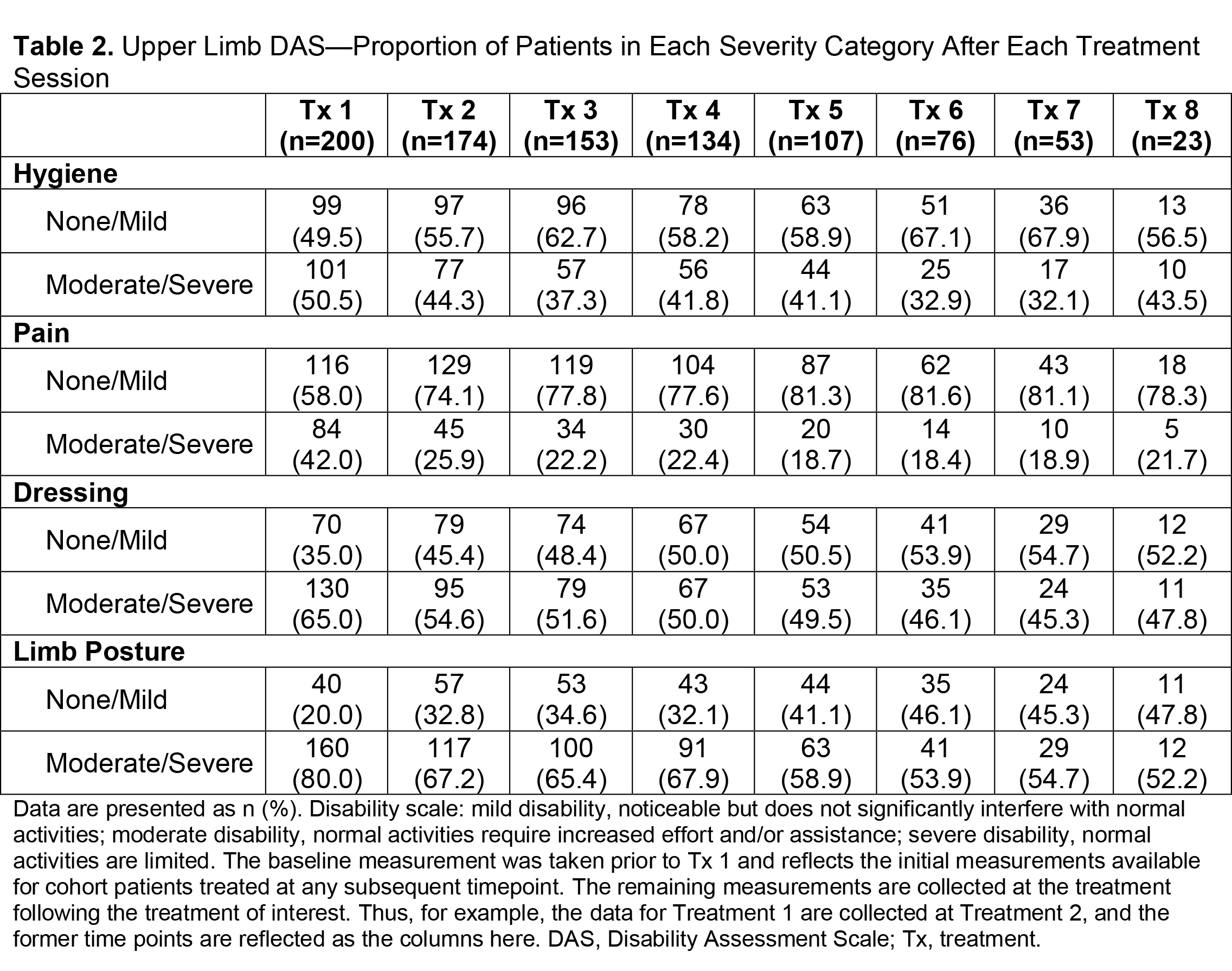
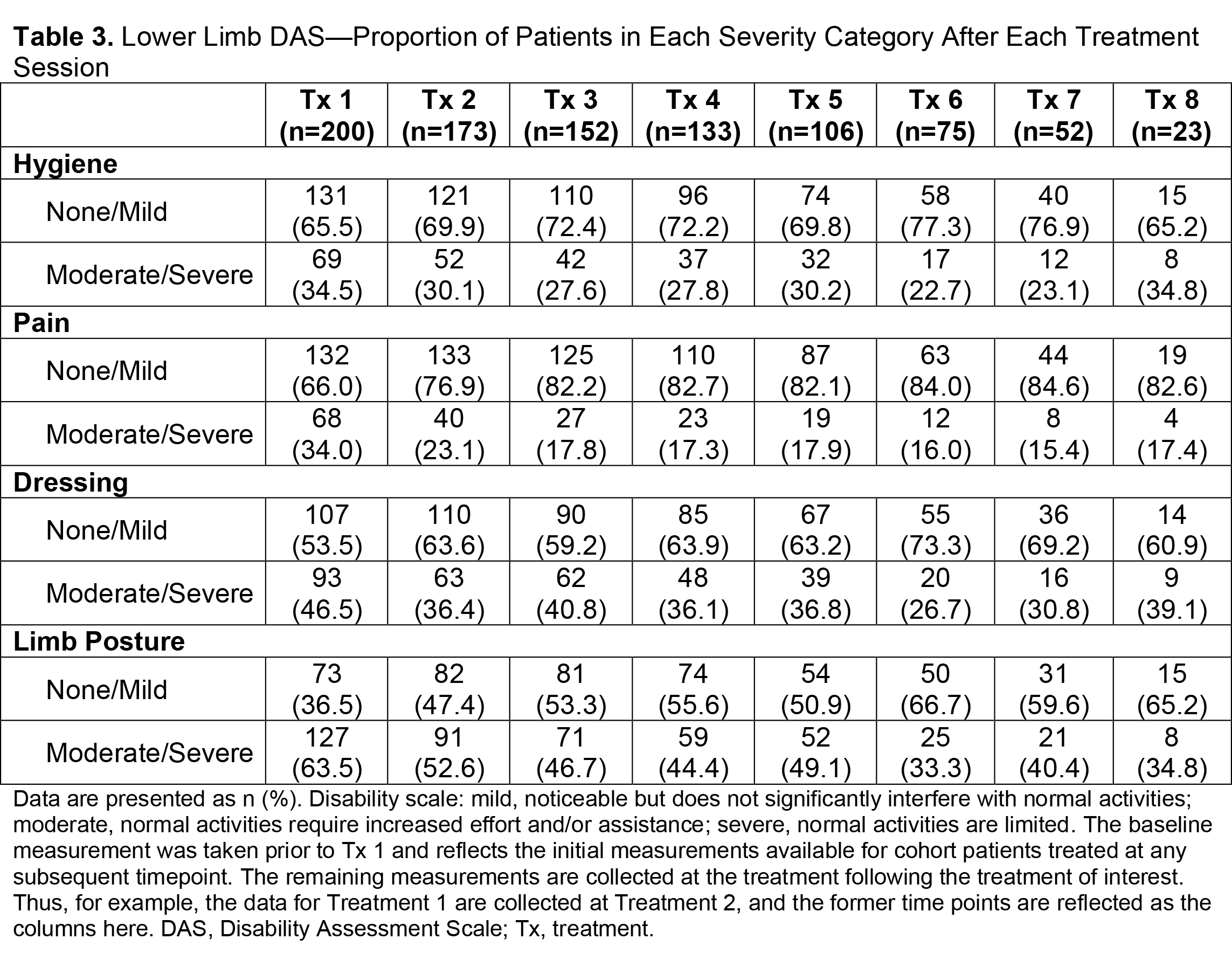
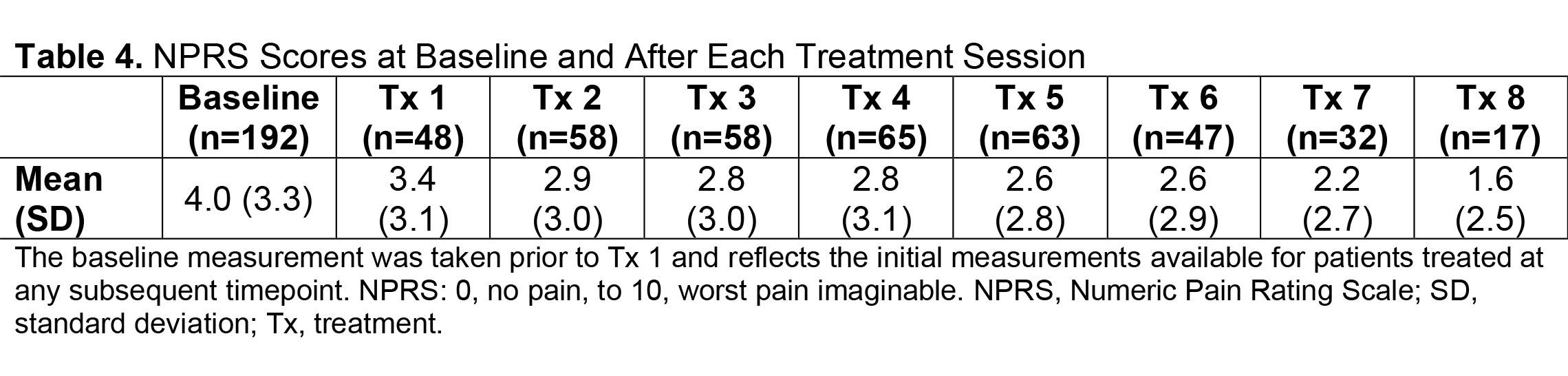
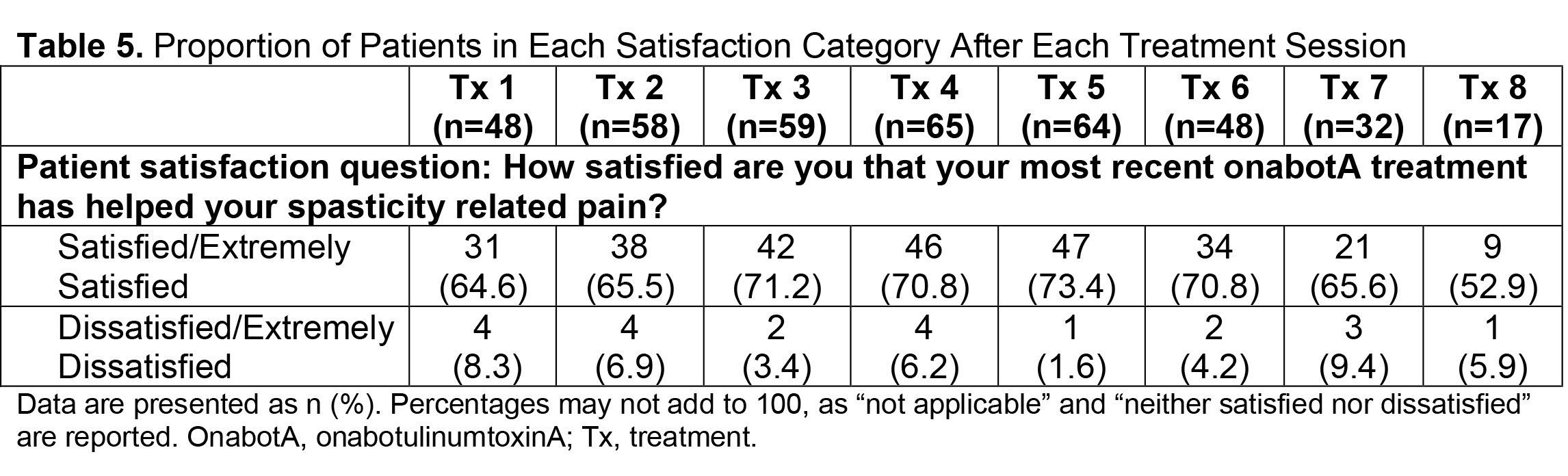
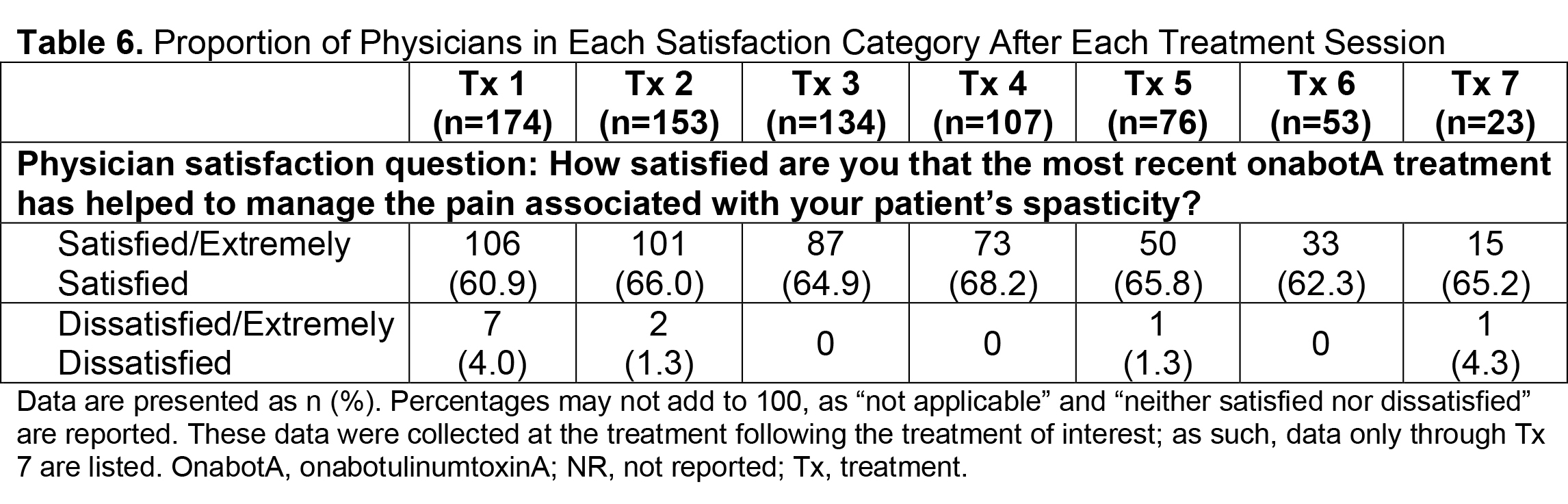
Results: Of 730 patients in the total analysis population of ASPIRE, 275 (37.7%) hemiparetic patients received treatment for both UL and LL during the same session and 200/275 (72.7%) of those patients were hemiparetic with PSS. The total mean (SD) onabotA dose per treatment session ranged from 430.8 (196.1) U to 522.4 (192.5) U [table1]. Per the DAS for UL and LL, the proportion of patients with no or mild disability generally increased or remained stable over time [table2], and the proportion of patients with moderate or severe disability generally decreased or remained stable over time [table3]. Mean (SD) NPRS scores decreased with every treatment session [table4]. Additionally, the majority of patients and physicians were satisfied/extremely satisfied with onabotA for pain management throughout the study [table5, table6].
Conclusion: In this exploratory analysis of ASPIRE, onabotA treatment reduced functional impairment and pain, as assessed by the DAS and NPRS, respectively, in hemiparetic patients with PSS.
To cite this abstract in AMA style:
G. Bavikatte, G. Francisco, W. Jost, A. Barichich, E. Duarte, M. Schwartz, T. Musacchio, A. Esquenazi. Pain and Disability Outcomes in Hemiparetic Patients With Post-Stroke Spasticity: Exploratory Analysis of the ASPIRE Study [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/pain-and-disability-outcomes-in-hemiparetic-patients-with-post-stroke-spasticity-exploratory-analysis-of-the-aspire-study/. Accessed February 5, 2026.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/pain-and-disability-outcomes-in-hemiparetic-patients-with-post-stroke-spasticity-exploratory-analysis-of-the-aspire-study/