Objective: To describe the characteristics and outcomes of advanced Parkinson’s disease (PD) patients grouped by treatment duration of levodopa-carbidopa intestinal gel (LCIG, carbidopa-levodopa enteral suspension[CLES], in the US).
Background: About 40% of patients experience ineffective symptom control including dyskinesia, wearing off, and motor fluctuations after 4 to 6 years of oral levodopa treatment. Data remain limited with long-term LCIG treatment during routine clinical practice.
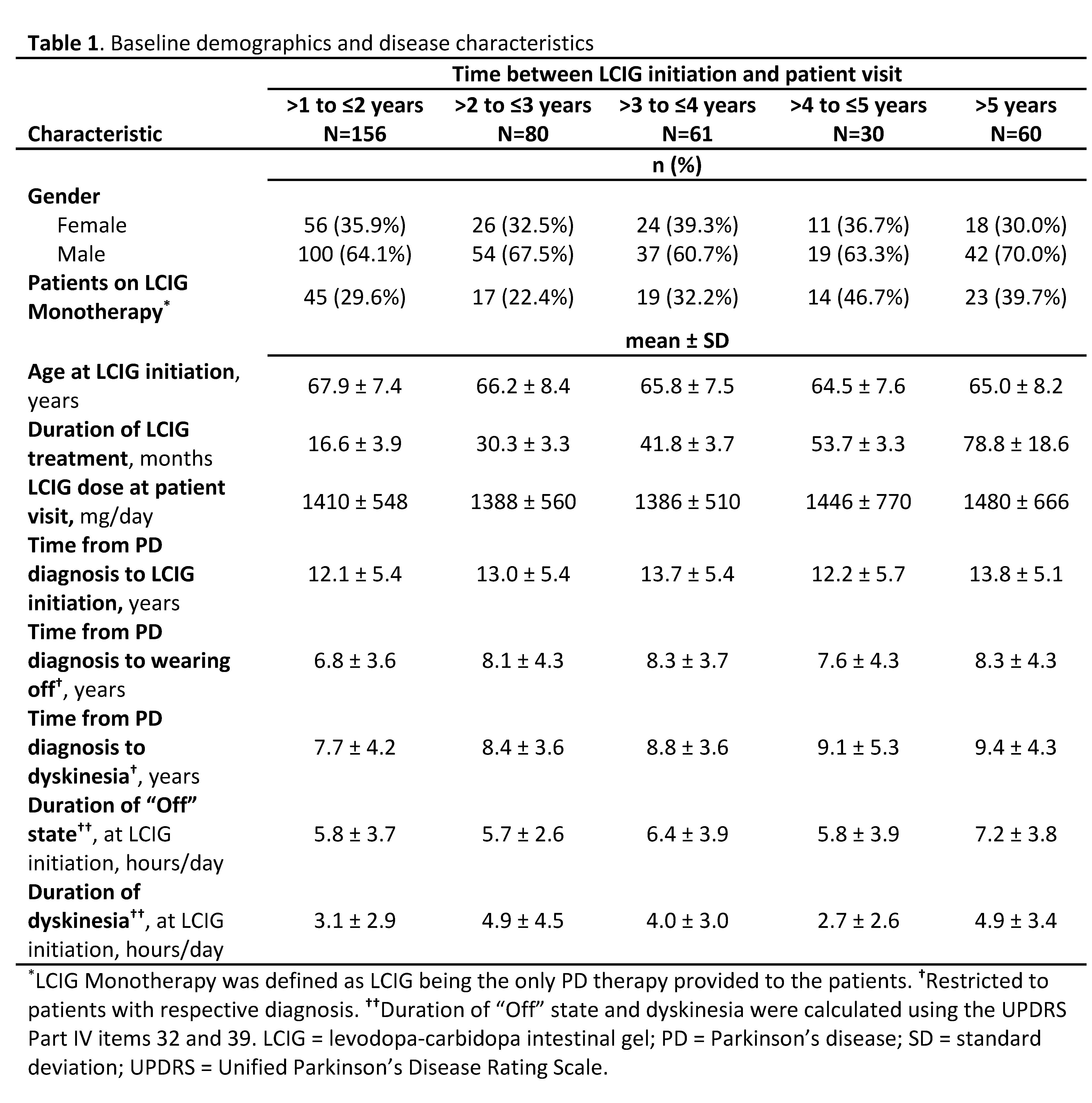
Method: COSMOS was a multi-country, retrospective, cross-sectional, post-marketing observational study(NCT03362879). Data were collected retrospectively at a single patient visit in advanced PD patients treated for at least 12 months with ongoing LCIG therapy. In this post-hoc analysis, patients(N=409) were stratified by the years of LCIG treatment duration at the patient visit: >1 to ≤2, >2 to ≤3, >3 to ≤4, >4 to ≤5, and >5 years. Assessments included patient demographics and disease characteristics, LCIG infusion settings, “Off” time and “On” time with dyskinesia (UPDRS Part IV: modified items 39 and 32), and dyskinesia severity (UPDRS IV: item 33; scores 0 [not disabling] to 4 [completely disabled]).
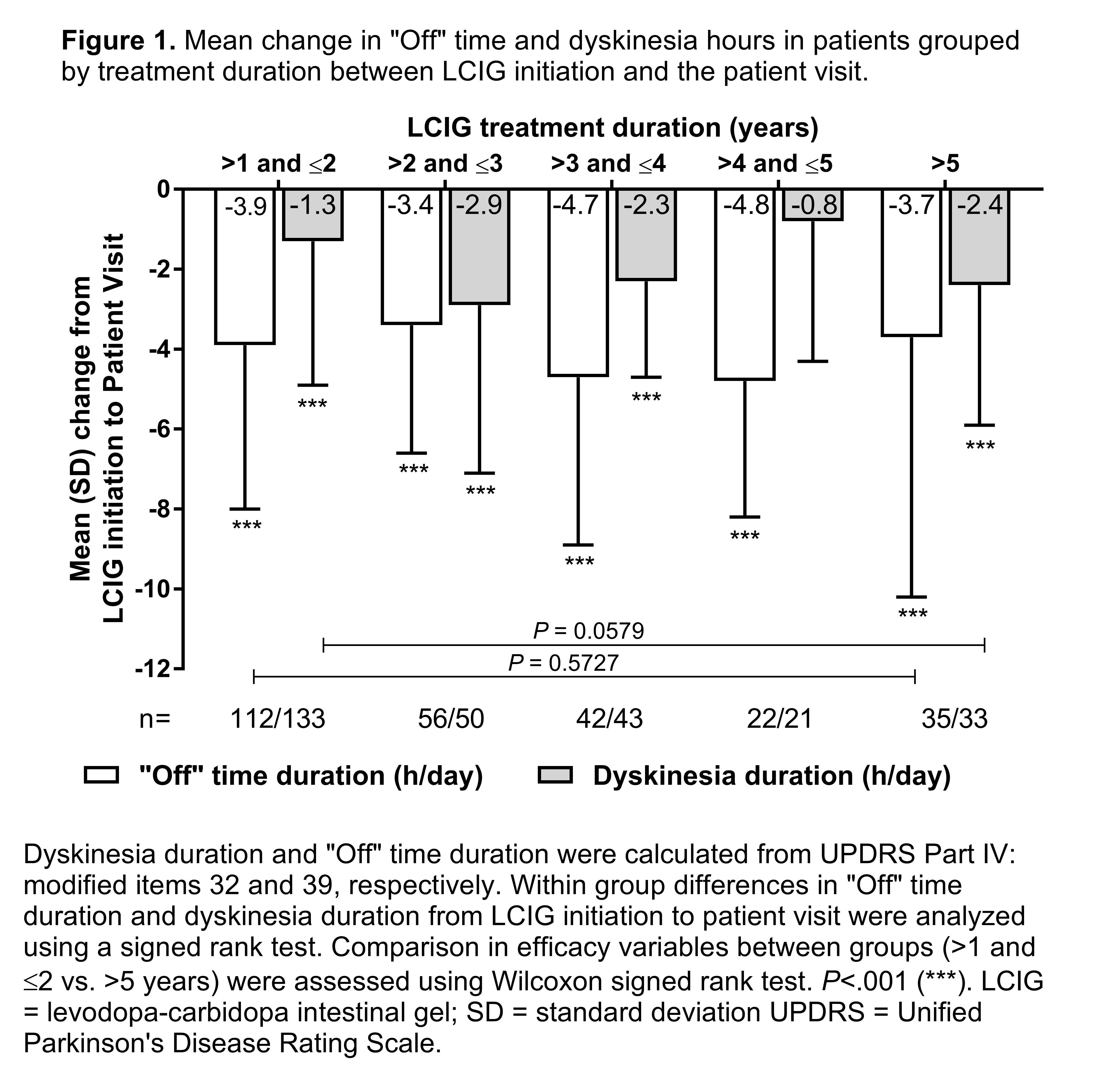
Results: At the patient visit, demographics were similar between patients independent of LCIG treatment duration: >3 to ≤4 (60.7% male; age 69.1 years), >4 to ≤5 (63.3% male; age 68.9 years), and >5 year (70.0% male; age 71.5 years) with additional disease characteristics presented in [Table 1]. “Off” time duration was significantly reduced at the patient visit compared to LCIG initiation in all of the LCIG duration subgroups [Figure 1]. Significant reductions in the duration of “On” time with dyskinesia were also observed in all but the >4 to ≤5 LCIG treatment duration subgroup [Figure 1]. Mean dyskinesia severity scores were reduced in patients with >5 years LCIG treatment from 1.8 at LCIG initiation to 1.1 at the patient visit (P =.0003); reductions were also observed in all other subgroups. The retrospectively collected safety events were in line with the known LCIG profile.
Conclusion: Although limited by the retrospective and post-hoc nature of the analysis, these data provide important information on the patients’ outcome profile with long-term LCIG treatment characterized by a sustained improvement in both “Off” time and dyskinesia symptoms.
To cite this abstract in AMA style:
A. Fasano, R. García-Ramos, T. Gurevich, R. Jech, S. Femia, J. Parra, M. Simu. Outcome Profiles in Advanced Parkinson’s Disease Patients Treated With Long-Term Levodopa-Carbidopa Intestinal Gel in a Routine Clinical Practice: Analysis From the COSMOS Study [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/outcome-profiles-in-advanced-parkinsons-disease-patients-treated-with-long-term-levodopa-carbidopa-intestinal-gel-in-a-routine-clinical-practice-analysis-from-the-cosmos-study/. Accessed January 21, 2026.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/outcome-profiles-in-advanced-parkinsons-disease-patients-treated-with-long-term-levodopa-carbidopa-intestinal-gel-in-a-routine-clinical-practice-analysis-from-the-cosmos-study/