Category: Parkinson's Disease: Neuroimaging
Objective: To optimize a neuromelanin-MRI (NM-MRI) protocol for robust and timely change analysis; and acquire serial NM-MRI data in controls and early Parkinson’s Disease (PD), to assess differences in depigmentation rates, associated with prognostic factors and subtypes.
Background: NM-MRI has emerged as the most promising non-invasive progression biomarker, which demonstrates changes in neuromelanin metrics commensurate with the expected loss of substantia nigra neurons [1-3]. This study intends to address a significant gap in the literature pertaining to a detailed longitudinal analysis of NM-MRI signal and volume changes in a large representative sample.
Method: This study will recruit 105 patients with PD, with matched controls, for 4 visits 6 months apart for imaging, blood biobanking, and assessments for MDS-UPDRS, cognition, and non-motor parameters using validated scales. Images will be acquired using a 3T MR system. The core protocol requires NM-MRI and MP-RAGE. MAGiC [4], DTI, fMRI and SWI sequences are acquired for exploratory endpoint analyses.
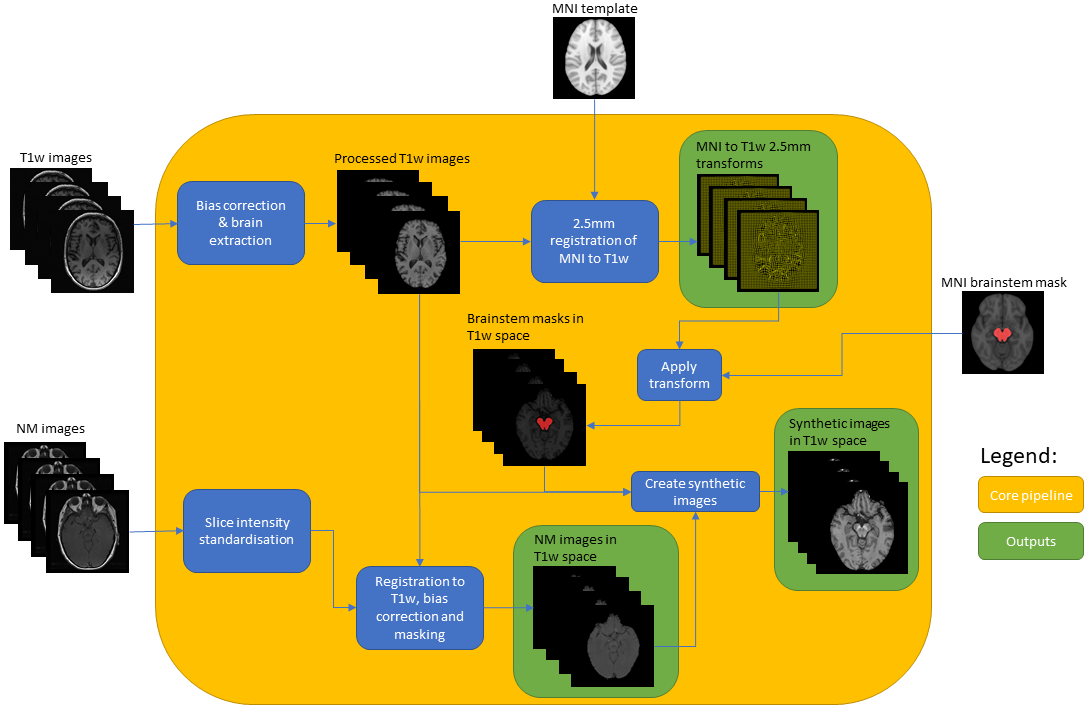
Results: Six different candidate NM-MRI sequences were acquired. The selection of the final NM-MRI scan was based on visual assessment over 4 subjects. A processing pipeline (Fig 1) computes a synthetic image generated from the NM-MRI and MP-RAGE sequences. Also, a synthetic template has been computed using the combined images from 39 controls transformed into MNI152 space using deformation fields resulting from registration of the corresponding MP-RAGE images to the 1mm T1-weighted template. This synthetic template allows a more precise registration of the subject’s synthetic sequence into MNI152 space. The synthetic image of each subject in MNI space was then used to compute measures of interest such as contrast to noise ratio, and new structural quantities related to disease progression.
Conclusion: We propose a robust 3T NM-MRI acquisition and novel processing pipeline, suitable for use in clinical MR systems. The pipeline has been successfully tested in healthy controls, with NM signal consistent with previously published data both from our group and others. We aim to encourage multi-center adoption and harmonization of data to facilitate future large-scale studies of PD progression.
References: [1] Schwarz ST, et al. T1-weighted MRI shows stage-dependent substantia nigra signal loss in Parkinson’s disease. Mov Disord. 2011 Aug 1;26(9):1633-8.
[2] Gaurav R, et al. Longitudinal Changes in Neuromelanin MRI Signal in Parkinson’s Disease: A Progression Marker. Mov Disord. 2021 Jul;36(7):1592-1602.
[3] Xing Y, et al. Neuromelanin-MRI to Quantify and Track Nigral Depigmentation in Parkinson’s Disease: A Multicenter Longitudinal Study Using Template-Based Standardized Analysis. Mov Disord. 2022 Feb 15.
[4] Tanenbaum LN, et al. Synthetic MRI for clinical neuroimaging: results of the magnetic resonance image compilation (MAGiC) prospective, multicenter, multireader trial. AJNR. 2017 38(6), pp.1103-1110.
To cite this abstract in AMA style:
T. Hayat, S. Pszczolkowski, Y. Xing, C. Tench, P. Morgan, J. Evans, D. Auer. Optimization of a neuromelanin MRI protocol for use in a longitudinal study of substantia nigra depigmentation in early Parkinson’s Disease (the InsIght-PD study) [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/optimization-of-a-neuromelanin-mri-protocol-for-use-in-a-longitudinal-study-of-substantia-nigra-depigmentation-in-early-parkinsons-disease-the-insight-pd-study/. Accessed December 19, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/optimization-of-a-neuromelanin-mri-protocol-for-use-in-a-longitudinal-study-of-substantia-nigra-depigmentation-in-early-parkinsons-disease-the-insight-pd-study/