Objective: This post-hoc analysis evaluated the effects of opicapone (OPC) treatment in patients with Parkinson’s disease (PD) who experience motor fluctuations and complications of therapy (CoT) at baseline when treated in real-world conditions.
Background: OPC proved to be effective in treating end-of-dose motor fluctuations in patients with PD [1,2]. The OPTIPARK study evaluated opicapone 50 mg in a heterogeneous population of patients treated in real-world conditions [3].
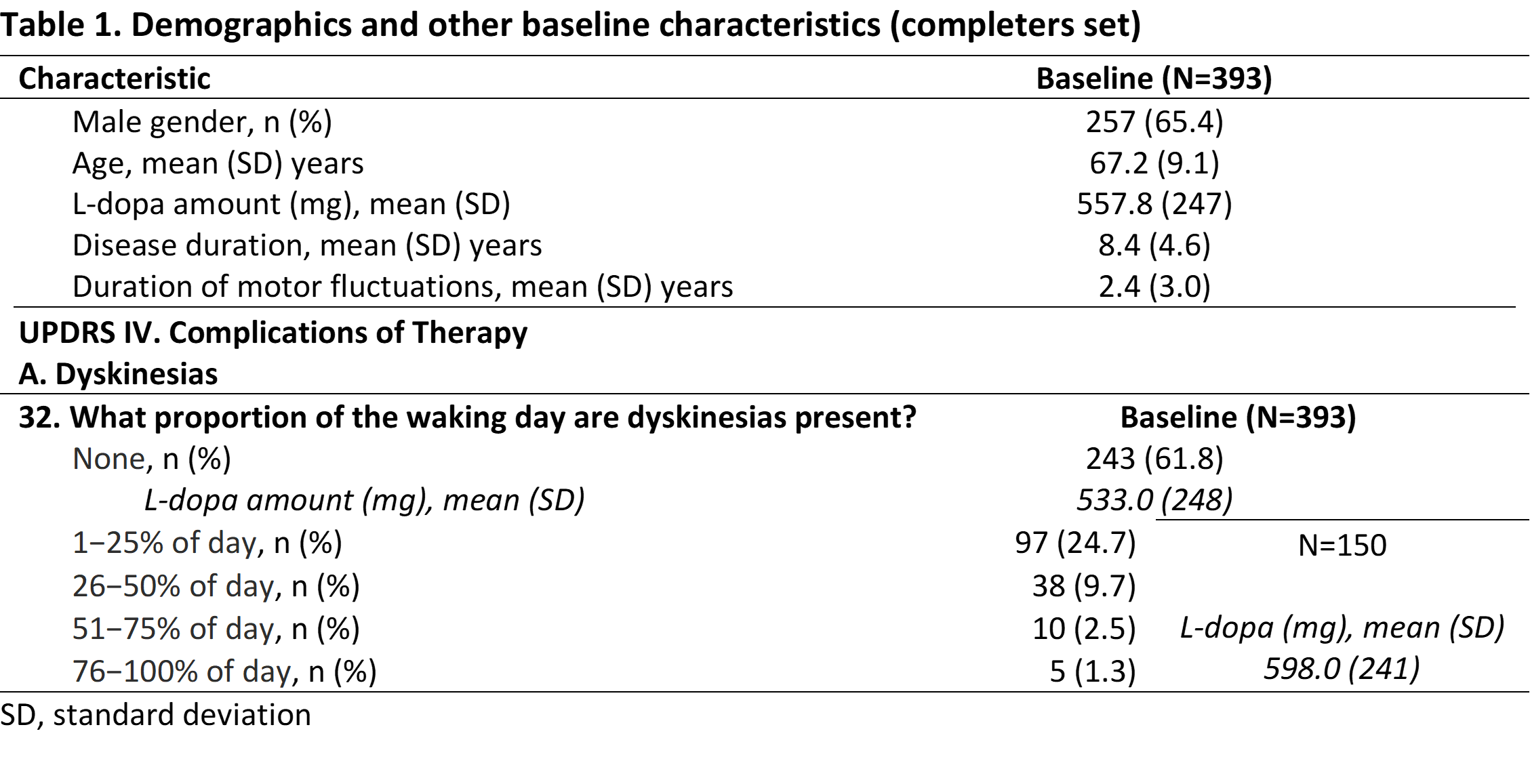
Method: OPTIPARK was a prospective, open-label, single-arm trial conducted in Germany and the UK. Patients with motor fluctuations received opicapone 50 mg in addition to current antiparkinsonian treatment. The primary efficacy 3-month endpoint was Clinician’s-Global-Impression-of-Change. Secondary assessments included Unified-Parkinson’s-Disease-Rating-Scale (UPDRS) and treatment-emergent adverse events (TEAEs). This post-hoc analysis evaluated the impact of OPC in patients who completed the study and reported CoT at baseline, assessed by UPDRS IV.A-32 (waking-day-dyskinesias [WdD]).
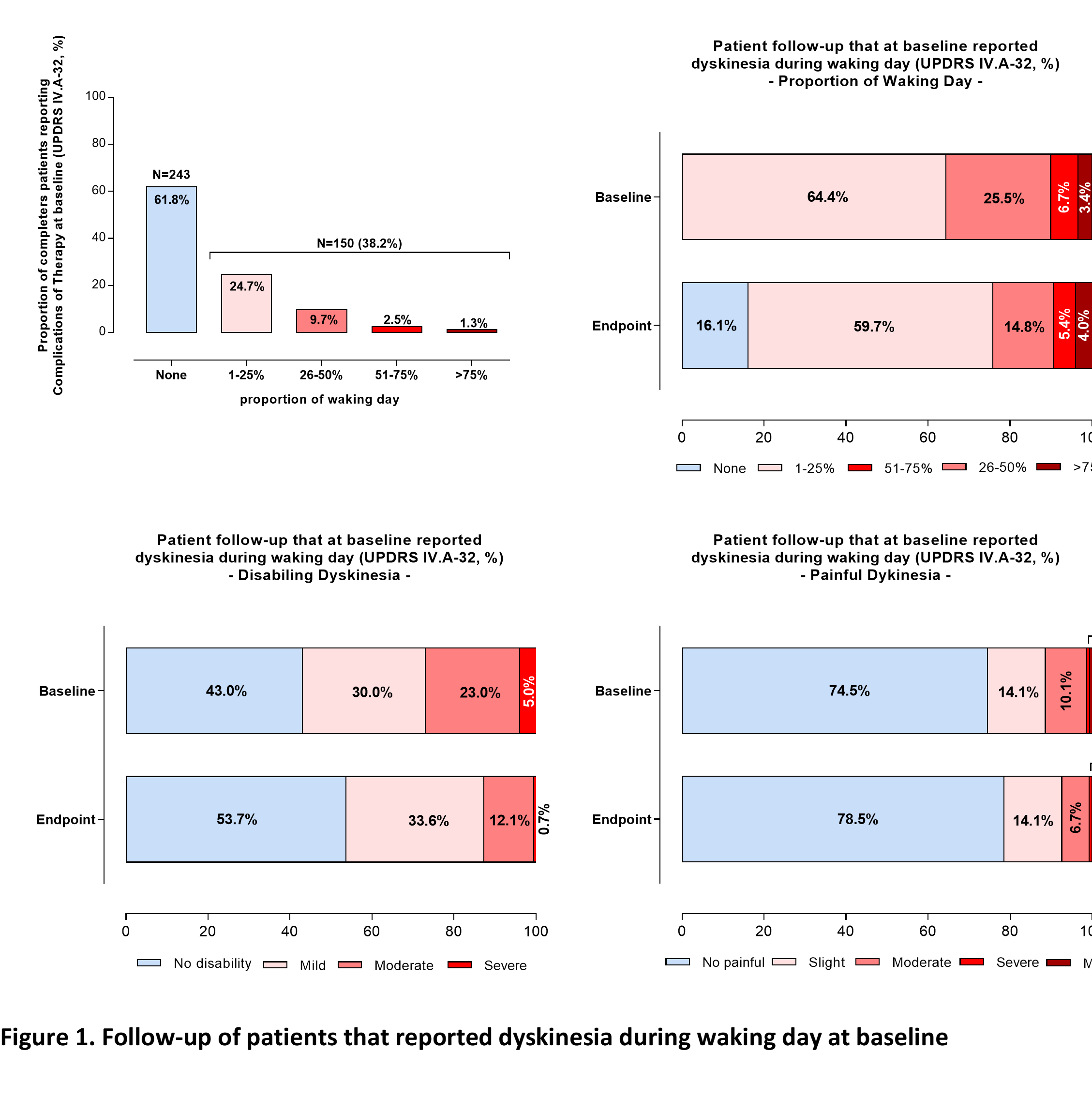
Results: 393 (82.4%) patients completed the 3-month endpoint (completers-set) and 150 reported WdD at baseline (Table 1). Most baseline WdD were not -or mildly- disabling and also not -or slightly- painful. Approximately 26% of these WdD completers reported dyskinesia as TEAE during the study. At endpoint, most of the patients (131 [88%]) demonstrated either maintained (54%) or improved (34%) WdD. Most remaining WdD were still not -or mildly- disabling and also not -or slightly- painful (Table 2, Figure 1). Notably, none worsened to either severe -or complete- disabling and severe -or marked- painful. Approximately 54% of dyskinesias reported as TEAE resolved following a mean daily levodopa decrease of ~67 mg (Table 2).
Conclusion: In clinical practice, opicapone was not associated with a clear WdD worsening in patients already reporting CoT; in fact, more than double of the patients reported improvement rather than worsening of WdD.
References: 1. Lancet Neurol. 2016;15:154–65. 2. JAMA Neurol. 2017;74:197–206. 3. Transl Neurodegener. 2020;9(1):9
To cite this abstract in AMA style:
H. Reichmann, A. Lees, M. Fonseca, D. Magalhães, J. Rocha, P. Soares-da-Silva. Opicapone in clinical practice in Parkinson’s disease patients with motor fluctuations and complications of therapy at baseline: findings from the OPTIPARK study [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/opicapone-in-clinical-practice-in-parkinsons-disease-patients-with-motor-fluctuations-and-complications-of-therapy-at-baseline-findings-from-the-optipark-study/. Accessed May 11, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/opicapone-in-clinical-practice-in-parkinsons-disease-patients-with-motor-fluctuations-and-complications-of-therapy-at-baseline-findings-from-the-optipark-study/