Session Information
Date: Monday, September 23, 2019
Session Title: Clinical Trials, Pharmacology and Treatment
Session Time: 1:45pm-3:15pm
Location: Agora 3 West, Level 3
Objective: To assess the onset of action for rimabotulinumtoxinBinjections in the salivary glands for the treatment of sialorrhea.
Background: Sialorrhea (drooling) causes significant morbidity including impaired oral hygiene, perioral irritation, embarrassment, swallowing impairment, and increased risk of aspiration. Local injections of botulinum toxin-B (BoNT-B) into the parotid and submandibular glands reduce saliva production. The onset of response is likely to be important in terms of patient and physician satisfaction with treatment.
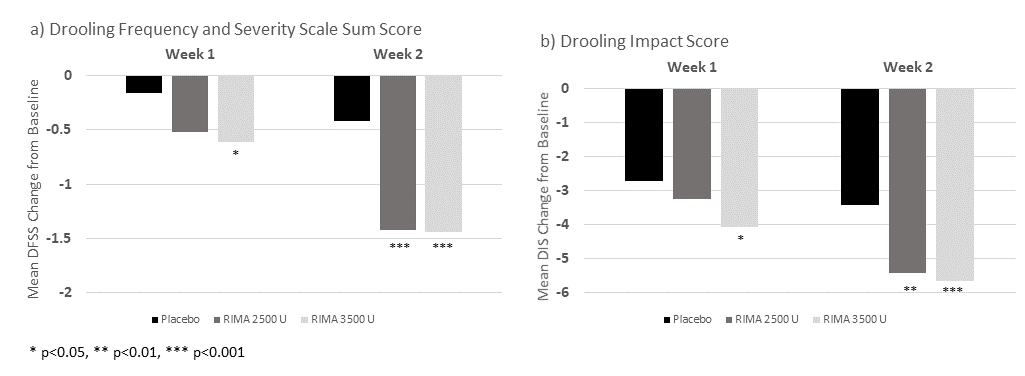
Method: This was a double-blind, placebo-controlled study conducted over 13 ±2 weeks. Adult patients with Parkinson’s disease, amyotrophic lateral sclerosis, medication-induced, stroke and other disorders were randomized (1:1:1) to receive rimabotulinumtoxinB 2500U vs 3500U vs placebo. Onset of RimabotulinumtoxinB efficacy was predefined as when the difference between the active dose vs placebo first became statistically significant (p <0.05) on the unstimulated salivary flow rate (USFR, co-primary outcome). Both clinicians and patients rated their global impressions of change (CGI-C, co-primary and PGI-C) at Weeks 1, 2, 4, 8 and 13±2. In addition, all patients self-completed the Drooling Impact Score (DIS) and Drooling Frequency and Severity Scale (DFSS).
Results: Reductions in USFR were observed with both rimabotulinumtoxinB doses compared with placebo, beginning at Week 1. A significantly greater proportion of patients had CGI-C and PGI-C ratings of “improved” or “very much improved” with RIMA 2500U compared with placebo, as early as Week 2. For the 3500U dose, significance on PGI-C versus placebo was noted as early as Week 1. Subject ratings of DFSS and DIS showed similar results to the primary outcome supporting a rapid onset [Figure1].
Conclusion: RimabotulinumtoxinB provides a rapid onset of efficacy for patients suffering with troublesome sialorrhea related to many neurological disorders.
To cite this abstract in AMA style:
L. Bahroo, S. Isaacson, R. Pahwa, D. Chary, T. Clinch, M. Lew. Onset of action for rimabotulinumtoxinB in the treatment of adult sialorrhea [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/onset-of-action-for-rimabotulinumtoxinb-in-the-treatment-of-adult-sialorrhea/. Accessed April 21, 2025.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/onset-of-action-for-rimabotulinumtoxinb-in-the-treatment-of-adult-sialorrhea/