Session Information
Date: Thursday, June 8, 2017
Session Title: Parkinson’s Disease: Clinical Trials, Pharmacology And Treatment
Session Time: 1:15pm-2:45pm
Location: Exhibit Hall C
Objective: Prospective non-interventional study conducted in routine clinical practice (PD0013; NCT02227355) to compare effectiveness and tolerability of rotigotine+levodopa (RTG+Ldopa) in Parkinson’s disease (PD) patients aged <70 or ≥70 years (yrs) who previously received Ldopa as monotherapy or combined with another dopamine agonist (DA).
Background: DAs are underused in elderly PD patients due to an association with increased side effects. Real-world studies can provide valuable data on effectiveness and tolerability of DAs in different age groups of patients treated in routine clinical practice.
Methods: Patients (Hoehn&Yahr [HY] 1–4) had received Ldopa for ≥6 months as monotherapy or in combination with another DA prior to starting RTG. Analysis was performed on patients aged <70 (“younger”) or ≥70 (“older”) yrs. Primary efficacy variable: change from Baseline to End of Observation (EoO ~6 months) in Unified Parkinson’s Disease Rating Scale (UPDRS) II. P-values are exploratory.
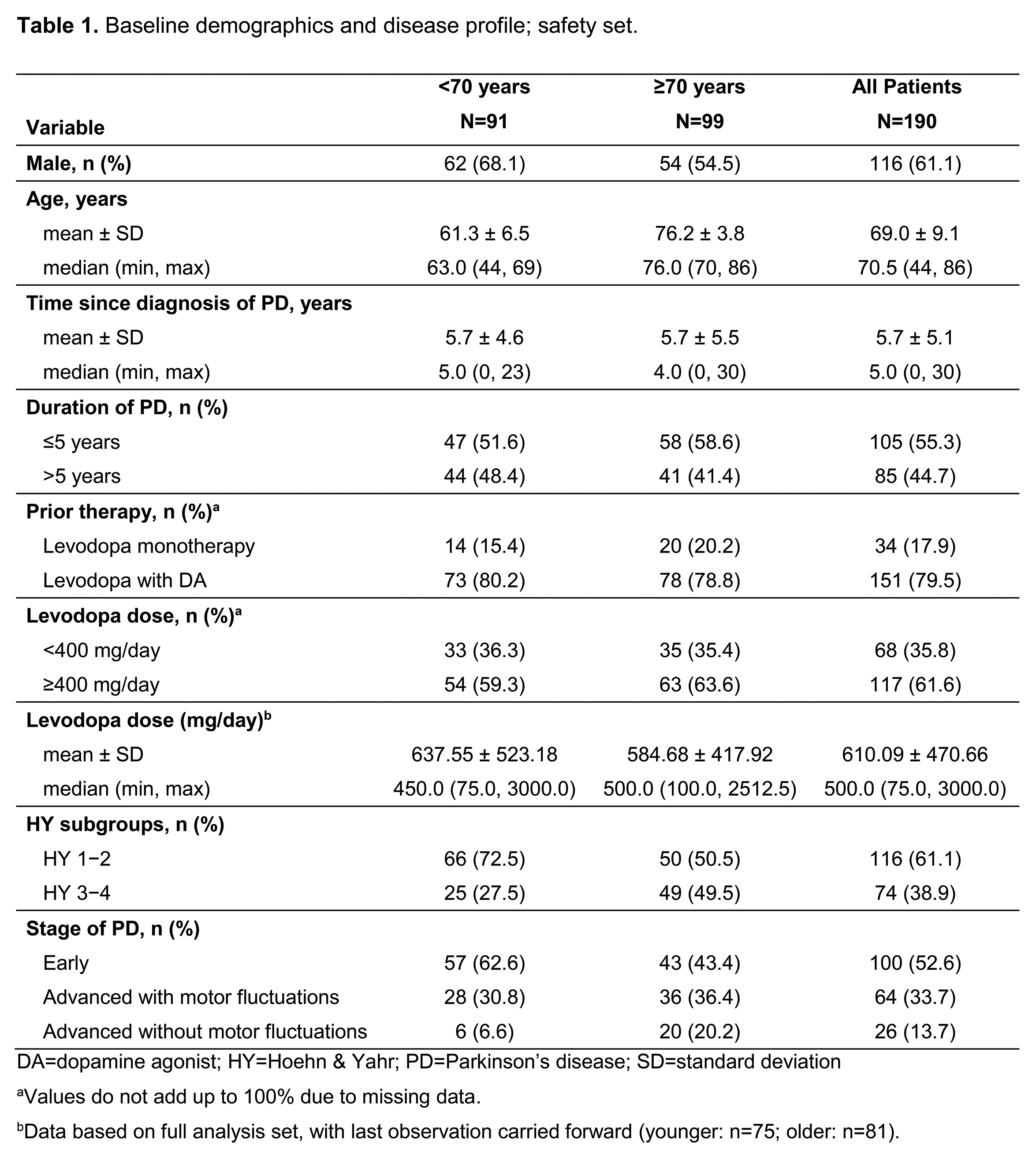
Results: Of 190 patients starting RTG (91 younger/99 older), 130 completed the study (68 younger/62 older). Most patients were switched from Ldopa+DA but addition of RTG as first DA was more common in older (20.2%) vs younger (15.4%) patients (Table 1). Mean±SD RTG duration (days) was 157.4±68.1 (younger) vs 143.6±83.4 (older). Mean±SD RTG exposure (mg/24h) was 6.1±3.4 (younger) vs 4.9±2.4 (older). Mean±SD change in Ldopa dose (mg/day) to EoO was minimal (+9.12±51.28 younger, 0.00±57.69 older).
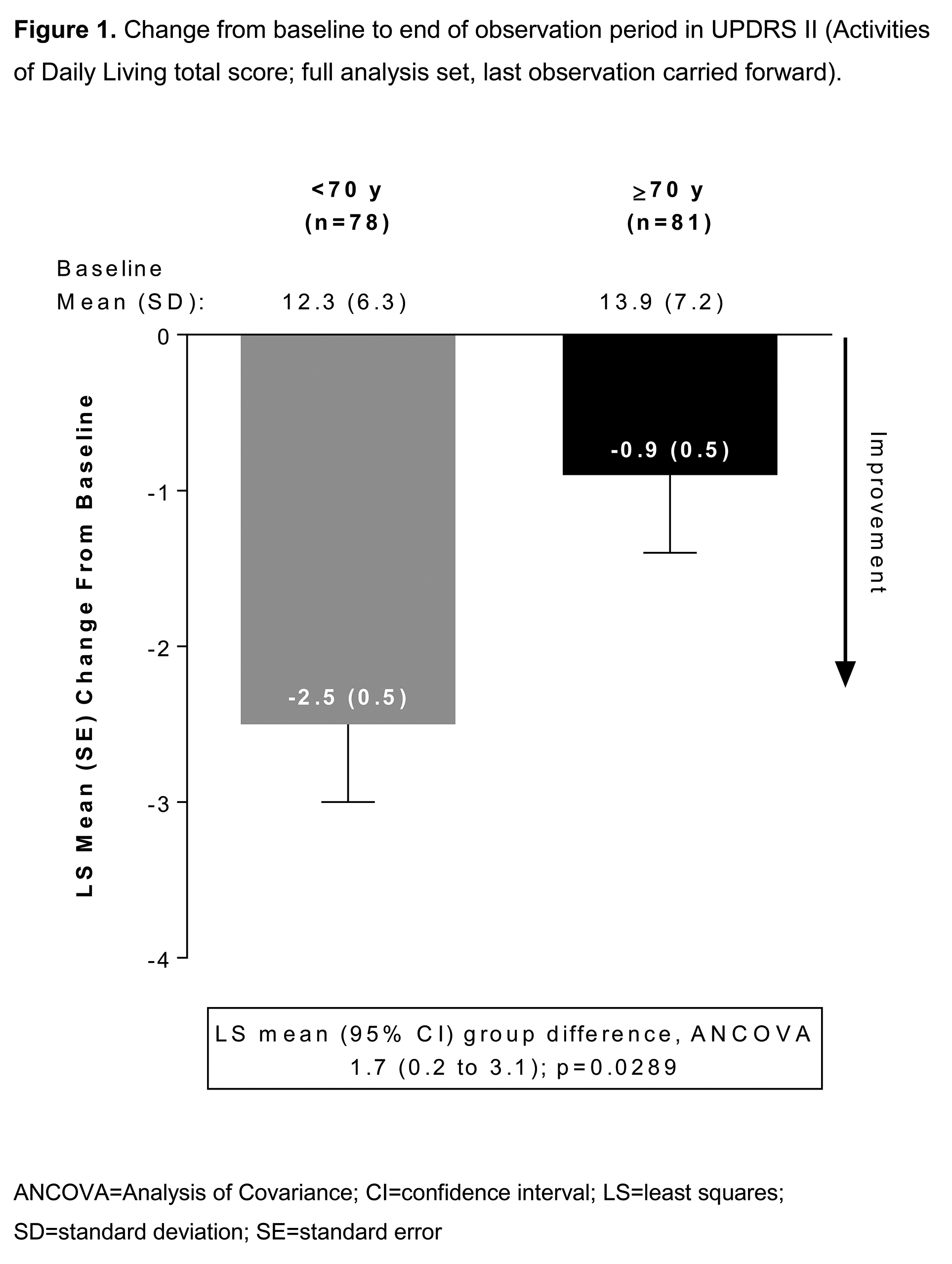
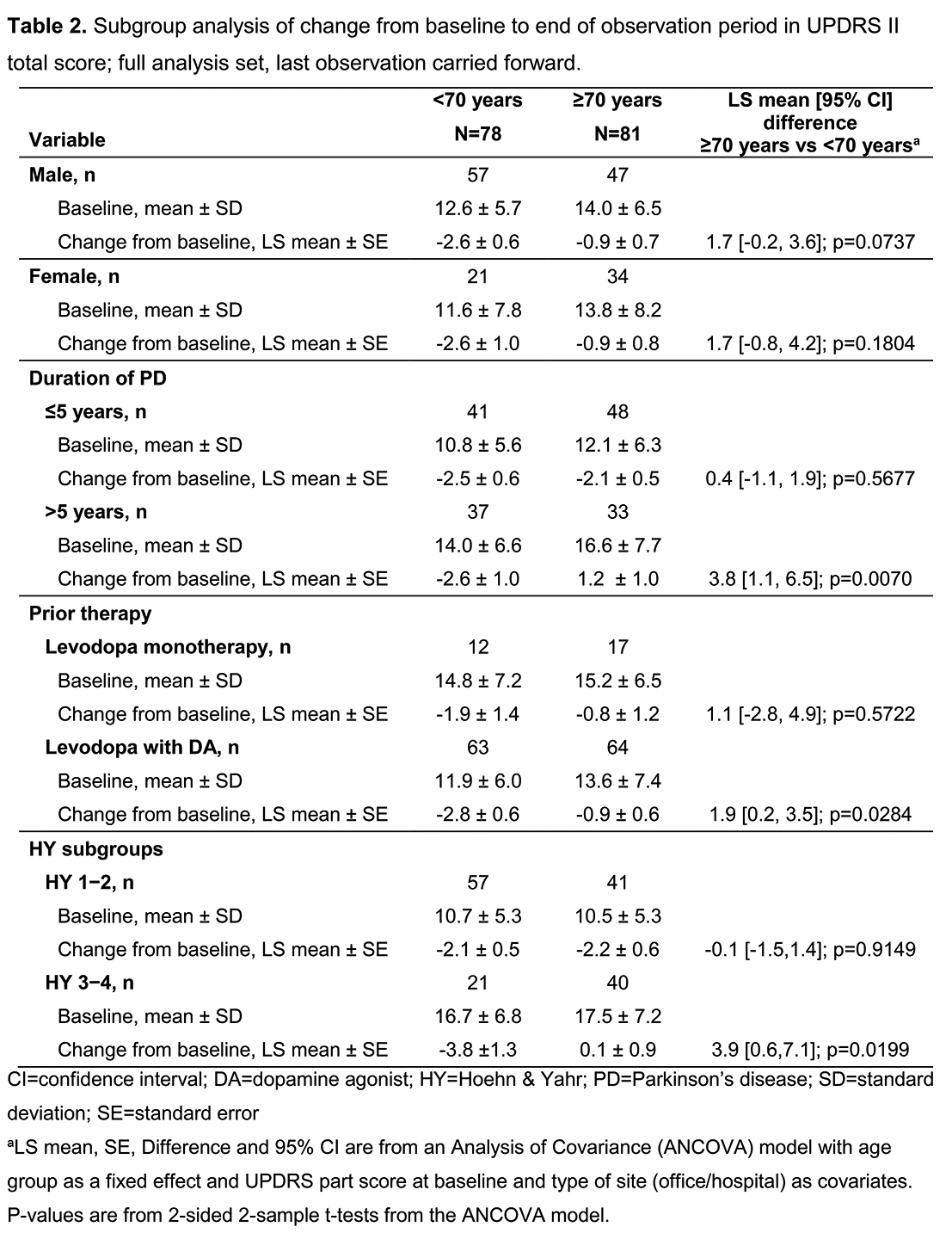
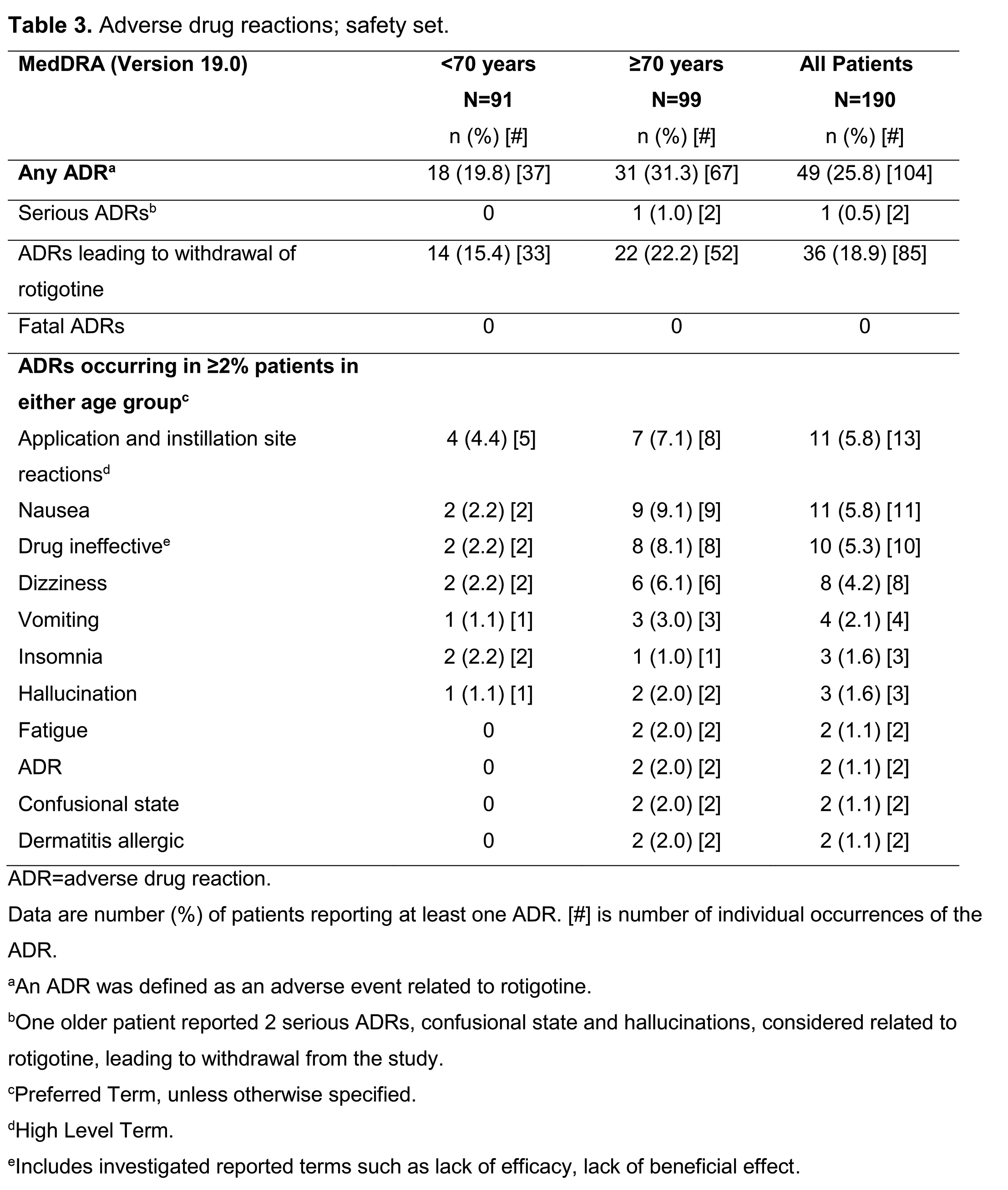
Improvement in UPDRS II was greater in younger patients (Fig 1) and in younger vs older patients at HY 3–4, with >5 yrs’ PD duration, or receiving Ldopa+DA combination at baseline (Table 2). UPDRS II improvement was similar in both age groups at HY 1–2, with ≤5 yrs’ PD duration, or receiving Ldopa monotherapy at baseline. There were more UPDRS II responders (≥20% decrease in UPDRS II) in the younger group (42.3% vs 25.9%; p<0.05). Changes in PD Sleep Scale 2 and Clinical Global Impressions-Item 2 responder rate were similar across age groups. In the older group, adverse drug reactions (ADRs) were more common and more patients discontinued due to ADRs (Table 3).
Conclusions: When added to Ldopa or switched from Ldopa+DA, RTG provided overall improvement to UPDRS II (activities of daily living), and was generally well-tolerated in both age groups (<70 or ≥70 yrs) in this large real-life study.
Funding: UCB Pharma
References: [table1]
[figure1]
[table2]
[table3]
To cite this abstract in AMA style:
D. Woitalla, M. Asgharnejad, L. Joeres, J.-C. Schuller, K.R. Chaudhuri. Observational study of the effectiveness and tolerability of rotigotine+levodopa therapy in older vs younger Parkinson’s disease patients [abstract]. Mov Disord. 2017; 32 (suppl 2). https://www.mdsabstracts.org/abstract/observational-study-of-the-effectiveness-and-tolerability-of-rotigotinelevodopa-therapy-in-older-vs-younger-parkinsons-disease-patients/. Accessed December 19, 2025.« Back to 2017 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/observational-study-of-the-effectiveness-and-tolerability-of-rotigotinelevodopa-therapy-in-older-vs-younger-parkinsons-disease-patients/