Category: Neuroimaging (Non-PD)
Objective: To determine more specific neuropathologies of the white matter in patients with multiple system atrophy-parkinsonian variant (MSA-P) using multi-shell diffusion magnetic resonance imaging (MRI) and myelin sensitive imaging techniques, which could be used to differentiate MSA-P and Parkinson’s disease (PD).
Background: MSA is clinically divided into parkinsonian variant and cerebellar variant. Pathologically, glial cytoplasmic inclusions (GCIs) are observed in the widespread regions of patient’s brain including white matter. The accumulation of GCIs has been known to cause chronic neuroinflammation that mainly occurred in oligodendrocyte. In recent years, advanced diffusion MRI methods, such as free water-eliminated diffusion tensor imaging (FWE-DTI) [1] or neurite orientation dispersion and density imaging (NODDI) [2], can capture neuroinflammation and more detailed alterations in brain microstructure than DTI, respectively. Moreover, magnetization transfer-saturation (MT-sat) imaging, which is myelin sensitive imaging technique, might be useful for evaluating the myelin content of white matter. [3]
Method: Age- and gender-matched 21 patients with MSA-P, 19 patients with PD and 20 healthy controls were enrolled. Multi-shell diffusion MRI and magnetization transfer saturation imaging were obtained on a 3-tesla Magnetic Resonance scanner. The measures of DTI, FWE-DTI, NODDI, and MT-sat were compared between groups using tract-based spatial statistics (TBSS) and region-of-interest (ROI) analyses.
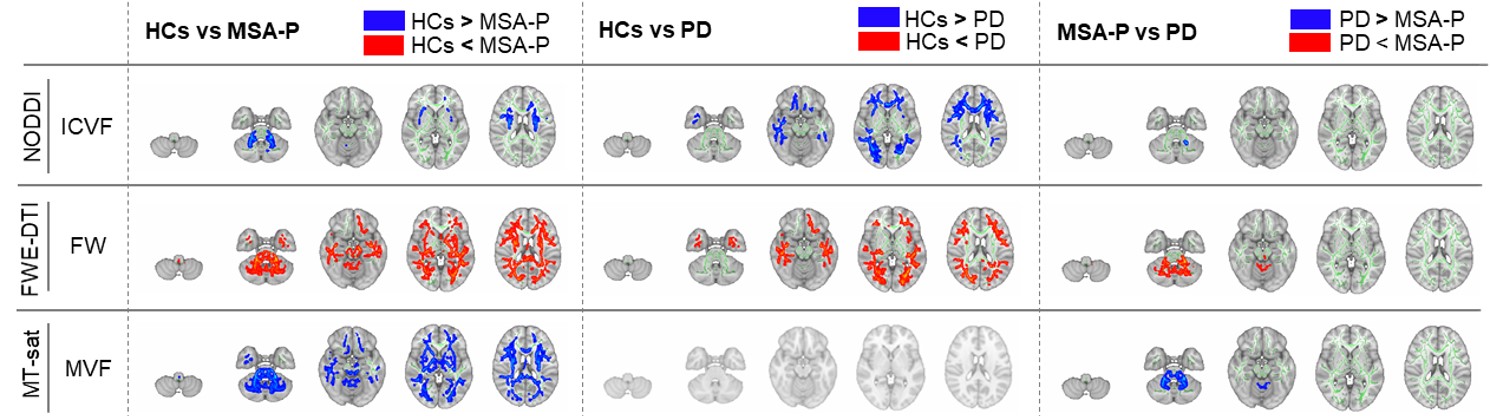
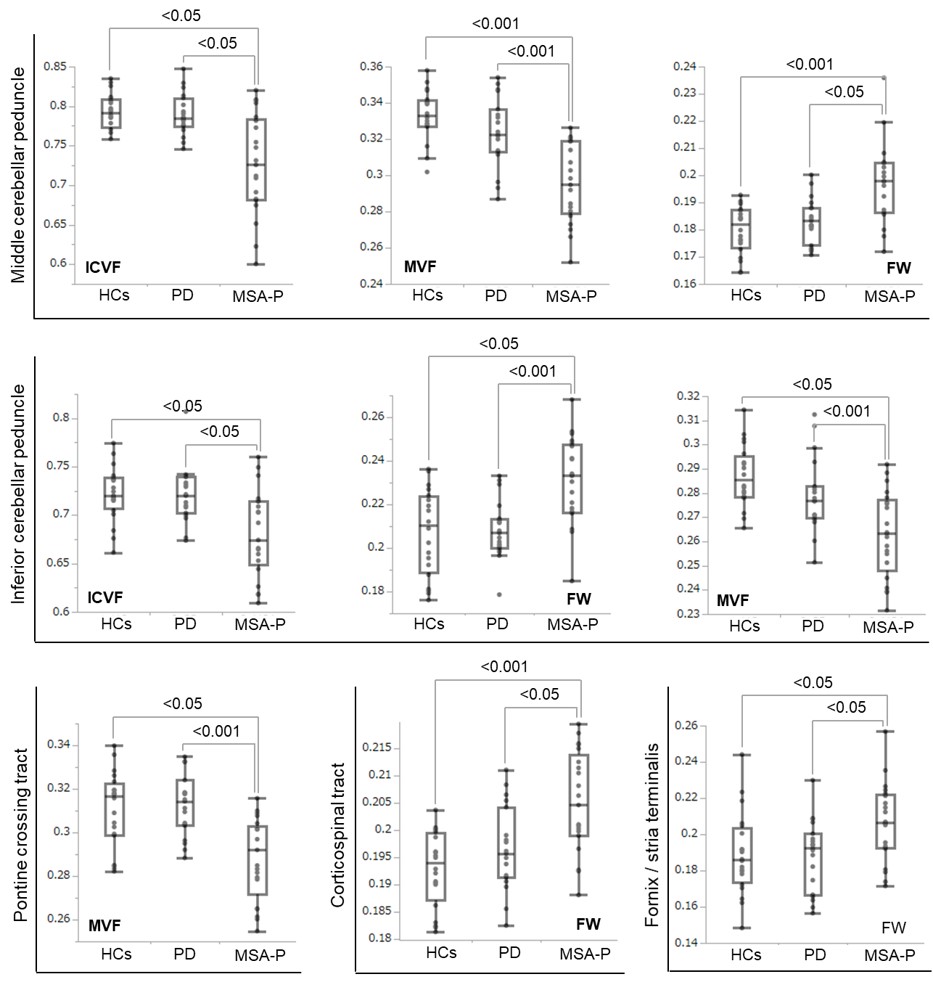
Results: TBSS revealed the limited alterations in free water fractional volume (obtained from FWE-DTI), myelin volume fraction (obtained from MT-sat) and intracellular volume fraction (obtained from NODDI) in the cerebral white matter, brain stem and cerebellum of MSA-P compared with those of PD [figure1]. The ROI analysis was also revealed that middle and inferior cerebellar peduncle, pontine crossing tract, corticospinal tract and fornix/stria terminalis were significantly altered in MSA-P [figure2].
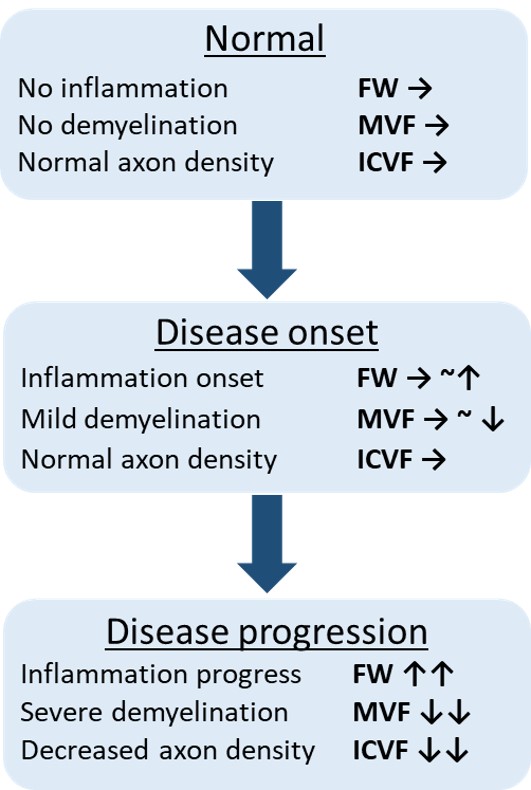
Conclusion: This study revealed the limited alterations in the middle and inferior cerebellar peduncle, pontine crossing tract, corticospinal tract. These findings were consistent with the pathological changes of neuroinflammation, axonal degeneration, and demyelination in MSA-P [figure3]. FWE-DTI, NODDI and MT-sat might be useful for diagnostic biomarker of atypical parkinsonism.
References: [1] Pasternak O, et al. Magn Reson Med 2009;62:717-730., [2] Zhang H, et al. Neuroimage 2012;61:1000-1016., [3] Duval T, et al. Funct Neurol 2016;31:217-228.
To cite this abstract in AMA style:
T. Ogawa, T. Hatano, K. Kamagata, C. Andica, H. Takeshige-Amano, W. Uchida, D. Kamiyama, Y. Shimo, G. Oyama, A. Umemura, H. Iwamuro, M. Ito, M. Hori, S. Aoki, N. Hattori. Neuroinflammation and Myelin changes in MSA-P evaluated using Advanced Diffusion MRI and Myelin Sensitive Imaging Techniques [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/neuroinflammation-and-myelin-changes-in-msa-p-evaluated-using-advanced-diffusion-mri-and-myelin-sensitive-imaging-techniques/. Accessed February 6, 2026.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/neuroinflammation-and-myelin-changes-in-msa-p-evaluated-using-advanced-diffusion-mri-and-myelin-sensitive-imaging-techniques/