Category: Parkinsonism, Atypical: PSP, CBD
Objective: The aim of this study was to determine how multimodal imaging biomarkers were related to abnormalities in three-dimensional motion-based gait and balance measurements in progressive supranuclear palsy (PSP).
Background: PSP is a neurodegenerative disorder characterized by tau inclusions and neurodegeneration in the midbrain, basal ganglia, thalamus, premotor and frontal cortex, measurable with PET and MRI, and by white matter degeneration of the dentatorubrothalamic tract, measurable with diffusion tensor imaging (DTI) [1-7]. Although PSP patients present with a variety of clinical syndromes, impairments in gait and postural instability are a key feature in most of them [8-13].
Method: We measured neuroimaging (MRI atrophy, DTI fractional anisotropy (FA) and mean diffusivity (MD), flortaucipir-PET uptake) and motion variables (global spatiotemporal variables: velocity, stride length, cadence, step width, total support and initial double support phases, gait stability ratio; postural imbalance; dynamic stability) on 19 participants with a clinical diagnosis of PSP. After regressing out age, statistical analyses were applied to describe gait and neuroimaging associations.
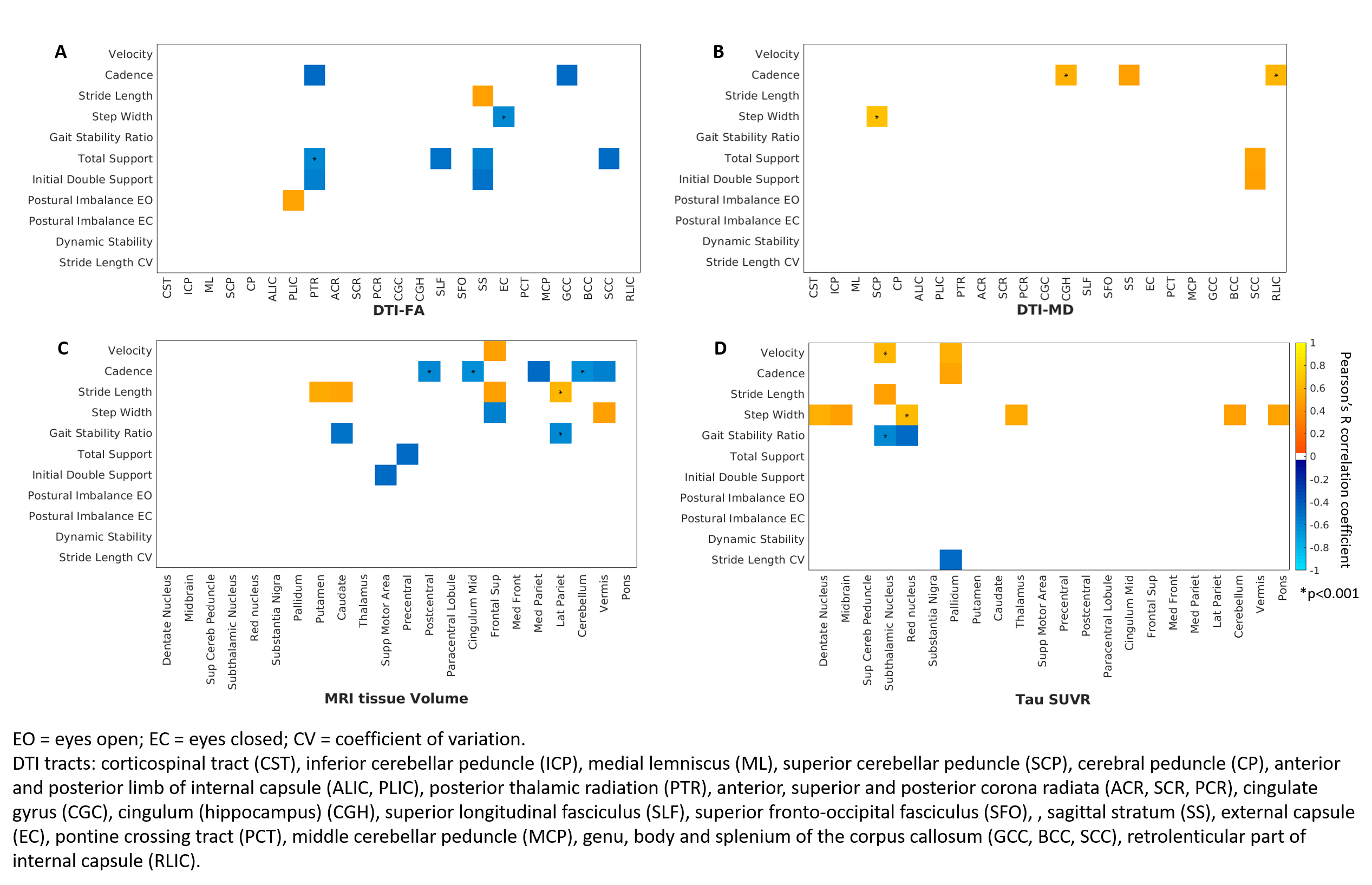
Results: PSP rating scale and its gait midline sub-scale were strongly correlated to gait abnormalities but not to postural imbalance [table1]. Pearson’s correlations showed that degeneration in white matter tracts like the superior cerebellar peduncle, corpus callosum and sagittal stratum and atrophy and flortaucipir-PET uptake in subcortical and fronto-parietal regions were associated with gait abnormalities such as reduced velocity and stride length and increased step width [figure1]. Principal component (PC) analysis on gait variables identified velocity, stride length, gait stability ratio and dynamic stability as the main contributors to PC1, which was related to larger frontal volumes and lower flortaucipir uptake in the precentral gyrus. PC2 was mostly related to cadence. Postural imbalance was the main contributor to PC3, which was related to higher flortaucipir uptake in the left paracentral lobule.
Conclusion: Although causality cannot be established, our study sheds light on the neurodegeneration of brain regions and white matter tracts that underlies gait and balance impairment in PSP, adding to the current understanding of these associations [14-16].
References: [1] Canu, E., et al., 2011. Diffusion tensor magnetic resonance imaging tractography in progressive supranuclear palsy. Mov Disord. 26, 1752-5. [2] Cope, T.E., et al., 2018. Tau burden and the functional connectome in Alzheimer’s disease and progressive supranuclear palsy. Brain. 141, 550-567. [3] Josephs, K.A., et al., 2013. Modeling trajectories of regional volume loss in progressive supranuclear palsy. Mov Disord. 28, 1117-24. [4] Whitwell, J.L., et al., 2011a. Disrupted thalamocortical connectivity in PSP: a resting state fMRI, DTI, and VBM study. Parkinsonism Relat Disord. 17, 599-605. [5] Passamonti, L., et al., 2017. F-18-AV-1451 positron emission tomography in Alzheimer’s disease and progressive supranuclear palsy. Brain. 140, 781-791. [6] Sintini, I., et al., 2019. Multimodal neuroimaging relationships in progressive supranuclear palsy. Parkinsonism & Related Disorders. 66, 56-61. [7] Whitwell, J.L., et al., 2011b. Clinical correlates of white matter tract degeneration in progressive supranuclear palsy. Arch Neurol. 68, 753-60. [8] Amano, S., et al., 2015. Discriminating features of gait performance in progressive supranuclear palsy. Parkinsonism & Related Disorders. 21, 888-893. [9] Egerton, T., Williams, D.R., Iansek, R., 2012. Comparison of gait in progressive supranuclear palsy, Parkinson’s disease and healthy older adults. Bmc Neurology. 12. [10] De Vos, M., et al., 2020. Discriminating progressive supranuclear palsy from Parkinson’s disease using wearable technology and machine learning. Gait & Posture. 77, 257-263. [11] Kammermeier, S., et al., 2018a. Postural Stabilization Differences in Idiopathic Parkinson’s Disease and Progressive Supranuclear Palsy during Self-Triggered Fast Forward Weight Lifting. Frontiers in Neurology. 8. 743. [12] Kammermeier, S., et al., 2018b. Qualitative postural control differences in Idiopathic Parkinson’s Disease vs. Progressive Supranuclear Palsy with dynamic-on-static platform tilt. Clinical Neurophysiology. 129, 1137-1147. [13] Raccagni, C., et al., 2018. Sensor-based gait analysis in atypical parkinsonian disorders. Brain and Behavior. 8. e00977. [14] Palmisano, C., et al., 2020. Gait initiation in progressive supranuclear palsy: brain metabolic correlates. NeuroImage: Clinical. 102408. [15] Zwergal, A., et al., 2011. Postural imbalance and falls in PSP correlate with functional pathology of the thalamus. Neurology. 77, 101-109. [16] Zwergal, A., et al., 2013. Functional disturbance of the locomotor network in progressive supranuclear palsy. Neurology. 80, 634-641.
To cite this abstract in AMA style:
I. Sintini, K. Kaufman, S. Loushin, H. Botha, P. Martin, M. Senjem, R. Reid, C. Schwarz, C. Jack, V. Lowe, K. Josephs, J. Whitwell, F. Ali. Neuroimaging associations to gait impairments in progressive supranuclear palsy [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/neuroimaging-associations-to-gait-impairments-in-progressive-supranuclear-palsy/. Accessed October 22, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/neuroimaging-associations-to-gait-impairments-in-progressive-supranuclear-palsy/